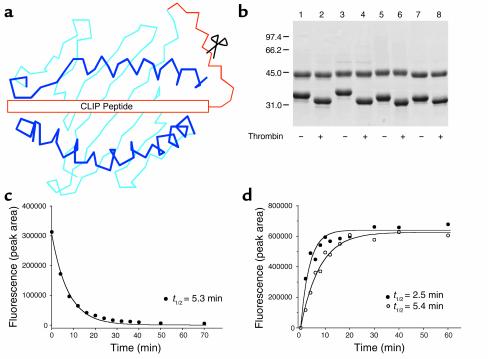
Figure 1.
Generation of tetramers from MHC class II/CLIP precursors. (a) Recombinant HLA-DR molecules were expressed in which the CLIP peptide was covalently attached to the N-terminus of the DRβ chain. These precursors were converted to the peptide-receptive form by thrombin cleavage of the linker. This strategy allowed a series of tetramers to be generated from a precursor protein. (b) SDS-PAGE (10%) of purified DR/CLIP precursors (7 μg per lane). Four different DR molecules were expressed in which DRα was paired with one of four DRβ chains (lanes 1 and 2: DRB1*0101; lanes 3 and 4: DRB5*0101; lanes 5 and 6: DRB1*1501; lanes 7 and 8: DRB1*0401). Thrombin cleavage of the linker (lanes 2, 4, 6, and 8) removed the CLIP peptide and reduced the molecular weight of the DRβ chain. (c) Kinetics of CLIP dissociation from DR4 (DRA, DRB1*0401). DR4 molecules were incubated with an Alexa-488–labeled CLIP peptide, and dissociation of labeled CLIP was examined at different time points following addition of a molar excess of unlabeled HA (residues 306–318) peptide by separating DR-bound and free peptide on an HPLC gel filtration column. (d) Peptide association is rapid and follows the kinetics of CLIP dissociation. DR4 molecules were incubated for different time intervals with Alexa-488–labeled HA peptide. The kinetics of CLIP dissociation (t1/2 of 5.3 min) closely mirrored those of HA peptide association (t1/2 of 5.4 min) indicating rapid binding of peptide. Addition of DM further accelerated this process (t1/2 of 2.5 min).