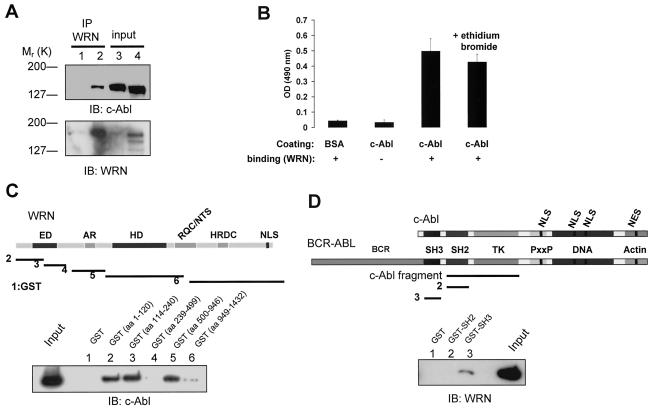
FIG. 1.
WRN interacts with c-Abl. (A) Lysates from WS (lanes 1 and 3) and control (lanes 2 and 4) fibroblasts were immunoprecipitated (IP) with polyclonal anti-WRN antibodies (lanes 1 and 2). The immunoprecipitates were analyzed by immunoblotting (IB) with anti-Abl and anti-WRN antibodies. Lysates containing 10% of the amount for immunoprecipitation were loaded as the input controls (lanes 3 and 4). (B) Direct interaction between WRN and c-Abl by ELISA. The purified full-length c-Abl was coated onto ELISA plates and followed by incubation with purified WRN. Rabbit antibodies against WRN (Ab200; Novus) were used to detect the bound WRN. Values are means ± standard errors (n = 3). OD, optical density; +, present; −, absent. (C) Known structural motifs and domains of WRN. ED, exonuclease domain; AR, acidic regions; HD, helicase domain; RQC/NTS, RecQ C-terminal/nucleolar targeting sequence; HRDC, helicase-related domain; NLS, nuclear localization signal. Bacterially expressed GST and GST-WRN fragments were bound to glutathione beads and incubated with 100 ng of purified c-Abl in the presence of ethidium bromide. After extensive washing, proteins were eluted, separated, and analyzed by IB with anti-Abl antibody. The input lane was loaded with 10 ng of c-Abl protein. (D) Schematic diagram of c-Abl domains in c-Abl and BCR-ABL proteins. TK, tyrosine kinase; PxxP, PXXP motif; DNA and actin, DNA- and actin-binding domains; NLS, nuclear localization signal; NES, nuclear export signal. The c-Abl fragment used for the in vitro kinase and WRN catalytic activity assays contains SH2 and TK domains. The binding assays were conducted as described except that 100 ng of WRN protein was used and followed by IB with anti-WRN antibody.