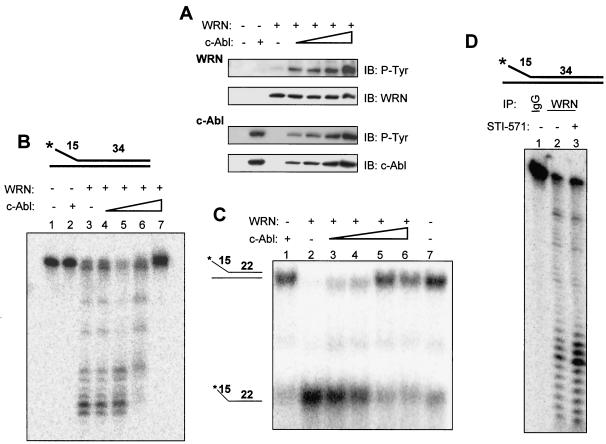
FIG. 6.
Tyrosine phosphorylation of WRN by c-Abl inhibits its exonuclease and helicase activities. (A) WRN was incubated with the c-Abl fragment by using the same protein concentrations and reaction conditions as those for the exonuclease assay. The proteins were analyzed by immunoblotting (IB) with anti-phosphotyrosine (P-Tyr) antibodies followed by anti-WRN or anti-Abl antibodies. (B) The structure of the exonuclease substrate is shown at the top. WRN (13 nM, lanes 3 to 7) was incubated in a 10-μl reaction volume with a 0, 0.02, 0.04, 0.09 (lanes 3 to 6), or 0.18 (lanes 2 and 7) nM concentration of the c-Abl fragment for 5 min at 28°C prior to the addition of the DNA substrate (0.5 nM, final concentration) under the conditions described in Materials and Methods, and products were analyzed on 14% denaturing polyacrylamide gels. (C) The structure of the helicase substrate is shown on the left. Purified WRN (1.0 nM, lanes 2 to 6) was incubated with the c-Abl fragment at a 0, 0.02, 0.04, 0.09 (lanes 2 to 5), or 0.18 (lanes 1 and 6) nM concentration in a 20-μl reaction volume for 5 min at 28°C prior to the addition of the DNA substrate (0.5 nM, final concentration) under the conditions described in Materials and Methods. Products were analyzed on 12% native polyacrylamide gels. Lane 7, no enzyme control. (D) Lysates from K562 cells with (0.6 μM, lane 3) or without (lanes 1 and 2) STI-571 treatment were immunoprecipitated with antibodies against immunoglobulin G (IgG) (lane 1) or WRN (lanes 2 and 3). The washed immunocomplexes were incubated with the exonuclease substrate (top, 0.5 nM, final concentration) as described. A representative was shown (n = 2). +, present; −, absent.