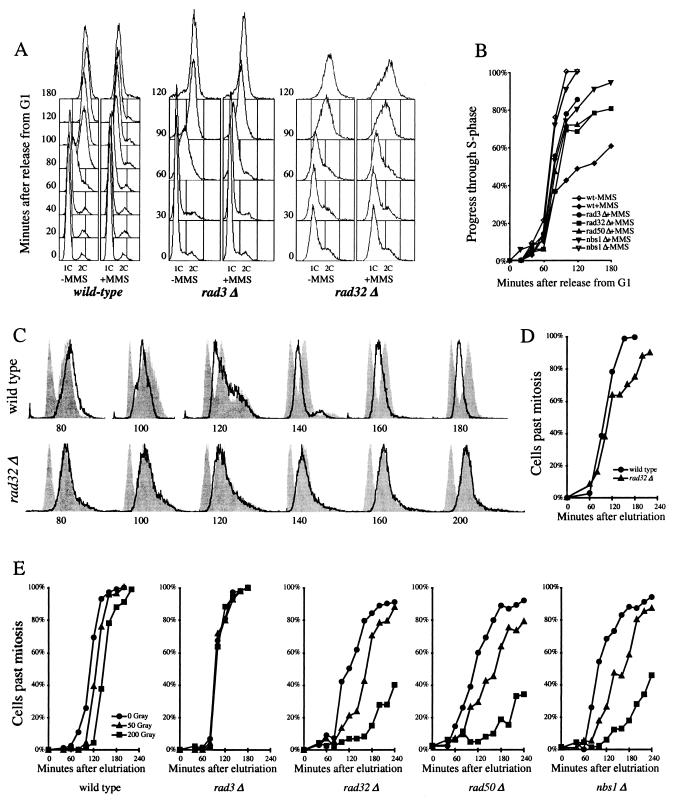
FIG. 5.
The MRN proteins are required for the S-phase DNA damage checkpoint but not the G2 DNA damage checkpoint. (A) Fluorescence-activated cell sorter (FACS) analysis of S-phase DNA damage checkpoint in cdc10-M17ts (EN2682), rad3Δ cdc10-M17ts (yFS260), and rad32Δ cdc10-M17ts (yFS263) cells. Synchronous G1 cultures were released from a G1 block in the presence or absence of 0.03% MMS. Progression through the S phase was determined by FACS. (B) Quantitation of the data in shown in panel A and data from rad50Δ cdc10-M17ts (yFS265) and nbs1Δ cdc10-M17ts (yFS267) cells. The percentage of progress through the S phase was calculated as the position of the mean of the FACS signal between the means of the 1C and 2C controls. (C) FACS analysis of S-phase DNA damage checkpoint in wild-type (PR109) and rad32Δ(TMN2799) cells. Elutriation-synchronized G2 cultures were treated with 0.01% MMS, a dose that does not activate the G2 DNA damage checkpoint but does activate the S-phase DNA damage checkpoint, and followed through mitosis and the S phase by FACS. For each time point, a separate set of FACS results is shown: the bold line represents the DNA content of the MMS-treated cells, the right-hand gray peak is a control consisting of the same time point of a parallel untreated culture, and the left-hand gray peak is a 1C control consisting of the 140-min sample of a parallel hydroxyurea-treated culture. (D) Division kinetics of the cells shown in panel C as determined by microscopically monitoring cell septation. (E) The response of wild-type (PR109), rad3Δ (NR1826), rad32Δ (TMN2799), rad50Δ (NR2841), and nbs1Δ (CC3223) cells to ionizing radiation during G2. Elutriation-synchronized G2 cultures were X-ray irradiated, and cells completing mitosis were identified by microscopically monitoring cell septation.