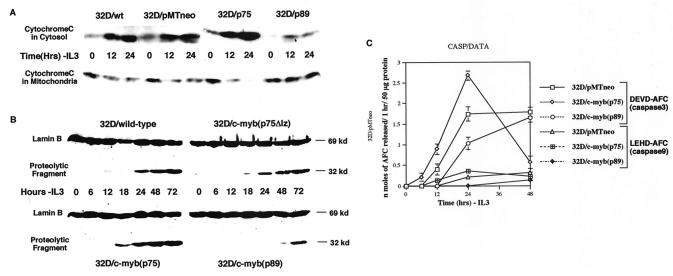
FIG. 4.
Analysis of caspase activity in p75-, p75Δlz-, and p89c-myb-transfected cells. (A) Cleavage of nuclear lamin B in wild-type, p75Δlz-c-myb, p75c-myb and p89c-myb cells subsequent to withdrawal of IL-3 from the medium. Cell lysates from different cell lines at the indicated time points were subjected to Western blotting with anti-lamin B antibody. The uncleaved lamin B is represented as a 69-kDa protein. A cleaved product of 32 kDa is detected secondary to lamin B breakdown by caspases. (B) Cytochrome c release during IL-3 withdrawal. Immunoblot analysis of cytosolic and organelle-bound fractions (as described in the text) after subjecting the various 32D cell lines to 0, 12, and 24 h of IL-3 withdrawal. The protein samples were analyzed by Western blotting with anti-cytochrome c monoclonal antibody. wt, wild-type; ev, empty vector. (C) Activation of DEVDase (caspase 3) and LEHDase (caspase 9) by IL-3 withdrawal. Empty-vector (pMTneo), p75c-myb and p89c-myb transfected cells were harvested at 0, 6, 12, 24, and 48 h following IL-3 withdrawal. After lysing the cells in Triton X-100-containing buffer as described in the text, the lysates were clarified by centrifugation, and the supernatants (50 μg of protein) were incubated with 50 μM substrate, including DEVD-AFC and LEHD-AFC, at 37°C for 1 h. Levels of released AFC were measured with a fluorescence microplate reader. Data are means of three experiments.