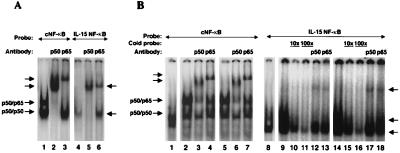
Figure 6.
Electrophoretic mobility shift assay using the IL-15 NF-κB motif GGGCTGGGGCTCCTCGATGTC and Ig κB NF-κB as consensus NF-κB (cNF-κB) AGTTGAGGGGACTTTCCCAGGC (where the underlined sequences are NF-kB binding site). (A) Extracts obtained from COS cells transfected with p65 and p50 expression constructs were used with cNF-κB (lanes 1–3) or IL-15 NF-κB (lanes 4–6). The typical p50–p65 heterodimer and p50–p50 homodimer can be readily seen with cNF-κB (lane 1). The IL-15 NF-κB forms a complex that comigrates with the p50–p50 homodimer of the cNF-κB as shown by an arrow (lane 4). Supershifts of this complex gave rise to two bands almost at the same height, as indicated by an arrow (lanes 5 and 6). (B) Five micrograms of the cellular extract from resting Jurkat (lanes 1 and 8), PHA/PMA-activated Jurkat (lanes 2–4 and 9–13), or ZnCl2-treated (Tax expressing) JPX-9 (lanes 5–7 and 14–18) were used in each reaction. The formation of the p50–p65 heterodimer and p50–p50 homodimer can be seen with cNF-κB and activated or Tax-expressing Jurkat cells. The p50 and p65 supershifts are marked for the cNF-κB probe. By using the IL-15 NF-κB probe, two species can be detected, as shown by the arrows. The specificity of the binding of these complexes was examined by a competition assay using a 10 and 100 times excess of the unlabeled IL-15 NF-κB probe added to the activated or Tax-expressing Jurkat extract (lanes 10, 11, 15, and 16). The p50 and p65 supershifts of these complexes resulted in the two bands that migrated almost at the same level as indicated by an arrow (lanes 12–13 and 17–18).