Figure 4.
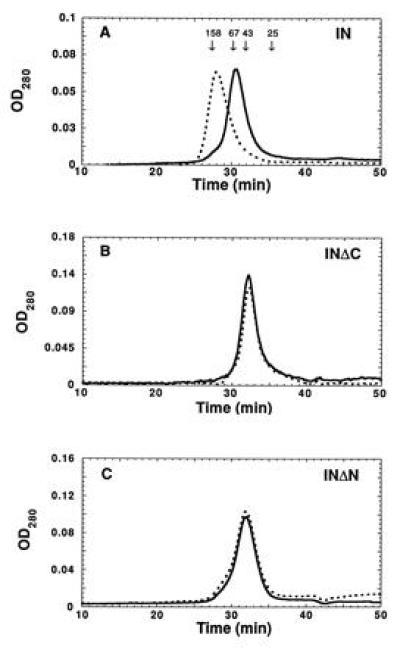
Zinc binding promotes tetramerization of HIV-1 integrase. (A) Gel filtration of integrase (IN1–288/F185K/C280S) on Superdex 200 after dialysis against buffer A containing EDTA (solid line) or zinc (dotted line). The zinc-dialyzed protein eluted at the expected position for tetramers (relative to globular protein standards). The EDTA-dialyzed protein eluted at the expected position for dimers. The peak elution times of chymotrypsinogen A (25 kDa), ovalbumin (43 kDa), bovine serum albumin (67 kDa), and aldolase (158 kDa) standards are indicated. (B) Integrase lacking the C-terminal domain (IN1–212/F185K) eluted exclusively as dimers after dialysis against EDTA or zinc. (C) Integrase lacking the N-terminal domain (IN50–288/F185K/C280S) eluted predominantly as dimers, with a trailing edge toward the tetramer position, after dialysis against EDTA or zinc.
