Figure 2.
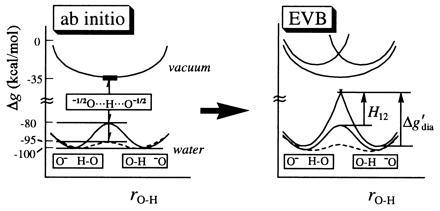
Demonstrating the effect of the environment on the nature of hydrogen bonding and the independence of our conclusions on the model used. The figure considers the HO− HOH system in vacuum and in water, representing the results of ref. 22 in a schematic way. Gas-phase calculations are presented for a single distance R = 2.4 Å between the oxygen atoms, while the calculations in solution are presented for both R = 2.8 Å (——) and for a least energy path where R is allowed to change upon displacement of the hydrogen (---). The distance R is short in vacuum, and the corresponding potential for proton motion is flat, reflecting the fact that H12 is similar in magnitude to Δg′dia. On the other hand, in polar medium, a barrier is induced because the concentrated charge of the O−1 H—O configuration is solvated more than the delocalized −½O···H···O−½ charge distribution. Now Δg′dia has a large solvent contribution and becomes larger than H12. Both the AI and the EVB calculations confirm that the short bond in vacuum is indeed strong and that in water a longer OHB is the most stabilized form. Furthermore, it is clear that the ordinary bond in water is more stable than the “strong” bond in vacuum.
