Figure 3.
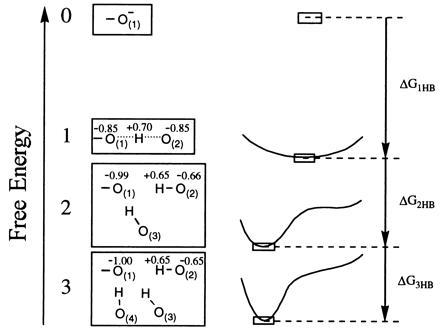
The effect of multiple hydrogen bonding on the energy of a negatively charged proton acceptor. While a single HB to O− will form an LBHB, successive HB donors pull it away from that configuration because of the larger stabilization provided by several OHBs, and the potential curve for proton movement takes on a double-well character. The numbers 0, 1, 2, and 3 correspond to the number of HB donors. The figure presents schematically the results of AI calculations with OH− and 0–3 water molecules. Note that the partial charges of hydrogens that are not explicitly shown are added to those of the shown oxygen atoms to which they are bonded.
