Figure 5.
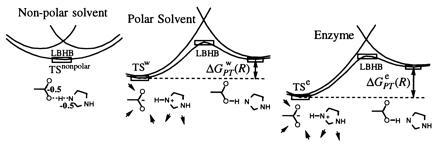
A schematic representation of the energetics of the Asp− HisH+ system (as a part of the TS in serine proteases) in different environments. In a nonpolar environment, the LBHB configuration [Asp− HisH+] ↔ [AspH His] is more stable than the Asp− HisH+ (OHB) configuration. However, in a polar solvent, the OHB is more stable than the LBHB configuration in any environment. The enzyme can interact strongly only with the polar [Asp− HisH+] configuration, and, since it has to stabilize the TS more than water does, it can only do this by pushing the energy of this state down. This increases ΔGPT and makes the LBHB configuration even less likely than in water. Note that the TS of the actual reaction is in the lowest point of the corresponding surface in our figure and not at its maximum. Also note that the TS is most stable in the enzyme and least stable in the nonpolar solvent.
