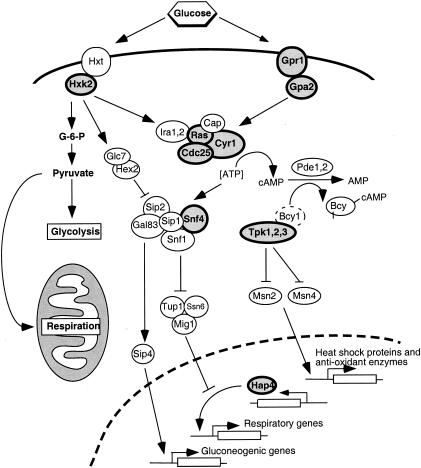
FIG. 7.
Glucose-sensing and signaling pathways of S. cerevisiae. Proteins indicated by bold type have been shown via genetic analyses to extend the replicative life span when either deleted or mutated. Glucose in the environment is first sensed by either of two complementary receptor families, the Hxt hexose transporters or the G-protein-coupled receptor, Gpr1. Glucose-mediated signals arising from these receptors cause Cdc25 to catalyze the formation of GTP-bound Ras1-Ras2, which then binds to and regulates the adenylate cyclase complex. Cyr1 (Cdc35) within the complex then generates cAMP, which in turn activates PKA, consisting of subunits Tpk1, Tpk2, and Tpk3. PKA inhibits Msn2 and Msn4, two factors required for the expression of heat shock proteins and superoxide dismutases, which are important for regulating the chronological life span. Further downstream, both pathways also converge on the Snf1 serine/threonine kinase complex, which is responsible for derepressing genes that are turned off during growth at relatively high glucose concentrations. The activity of the Snf1 complex is modified by the Glc7-Hex2 complex and also perhaps by a proposed kinase(s) that may be responsive to intracellular ATP and AMP levels. The Snf1 complex inhibits the Mig1-Ssn6-Tup1 repressor complex, required for shutting off the transcription of genes needed for respiration, and activates Sip4, which turns on the transcription of genes required for gluconeogenesis under conditions of low glucose concentrations.