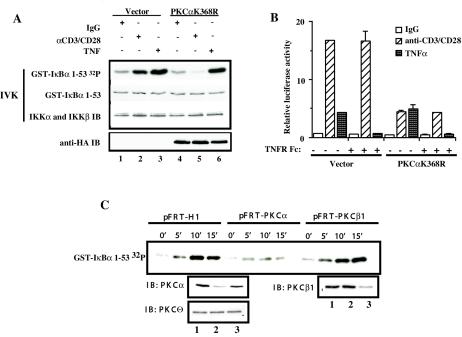
FIG. 2.
PKCα is required for CD3/CD28-induced activation of IKK complex and NF-κB-dependent transcriptional activity. (A) Jurkat T cells (10 × 106 per sample) were electroporated with 20 μg of catalytically inactive HA-PKCαK368R (lanes 4 to 6) or control vector pEF-BOS (lanes 1to 3). Eighteen hours later, transfected Jurkat T cells were cross-linked with anti-CD3 and anti-CD-28 antibodies (lanes 2 and 5) or were stimulated with TNF-α as a control (lanes 3 and 6). IKK activity was measured in an IVK assay using glutathione S-transferase (GST)-IκBα(1-53) as a substrate (GST-IκBα 32P). Coomassie staining of the polyvinylidene difluoride membrane containing the IVK indicates equal amounts of IκBα substrate (GST-IκBα), and immunoblotting (IB) for IKKα and β indicates equal amounts of complex immunoprecipitation per lane. Expression of HA-PKCαK368R was confirmed by immunoblotting with anti-HA antibodies (anti-HA IB). (B) Jurkat T cells (2 × 106 per sample) were transfected in duplicate with 2.4 μg of catalytically inactive HA-PKCαK368R or 2.4 μg of control vector pEF-BOS together with reporter κB-luc (0.38 μg) and Tk-Renilla (0.02 μg) plasmids by the FuGENE6 method. Twenty hours later, transfected Jurkat T cells were cross-linked with anti-CD3 and anti-CD28 antibodies or were stimulated with TNF-α. TNFR Fc fusion protein was added at the moment of TNF-α stimulation at 1 μg/ml. Four hours later, cells were harvested and luciferase activities were measured. The κ[/beta]-luc activity was normalized to Renilla luciferase activity (relative luciferase activity). (C) Jurkat T cells (10 × 106 per sample) were electroporated with 40 μg of pFRT-PKCα (lane 2), pFRT-PKCβ1 (lane 3), or pFRT control vector (lane 1). Forty-eight hours later, the transfected cells were treated with anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) antibodies for 45 min on ice and cross-linked with goat anti-mouse antibodies in solution at 37°C for the indicated periods of time. IKK activity was measured in IVK assay as described above. Protein levels following the suppression of endogenous PKCα or PKCβ1 expression were confirmed by immunoblotting. Equal amounts of protein per lane were demonstrated by immunoblotting with anti-PKCθ antibodies.