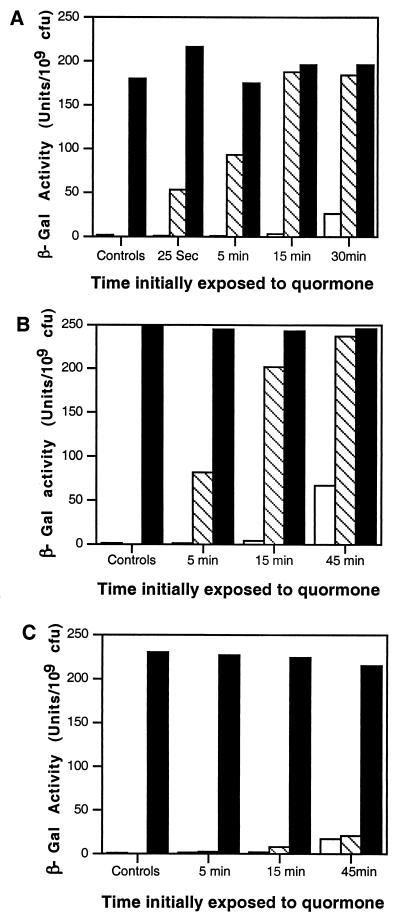
FIG. 2.
In situ activation of TraR by acyl-HSLs. 3-Oxo-C8-HSL (25 nM final concentration) (A), 3-oxo-C6-HSL (100 nM final concentration) (B), or C6-HSL (2.5 μM final concentration) (C) was added to exponential-phase cultures [OD600 = 0.15 (ca 107 CFU/ml)] of A. tumefaciens strain NTL4(pZLR4) growing in ABM minimal medium containing IPTG to induce expression of traR. The cultures were incubated, 4-ml samples were withdrawn at the indicated times, and the cells were harvested and washed extensively as described in Materials and Methods. The washed cells were resuspended in 4 ml of ABM lacking IPTG, and each culture was split into three subcultures. One subculture was assayed for β-galactosidase activity, expressed as unitsper 109 CFU, immediately after washing and resuspension (open bars). 3-Oxo-C8-HSL or 3-oxo-C6-HSL was added to the second subculture (solid bars), and an equal volume of sterile distilled water was added to the third subculture (hatched bars). The subcultures were reincubated in parallel with vigorous shaking for 4 h, at which time the cells from each culture were collected by centrifugation and assayed for β-galactosidase activity. Parallel cultures, not subjected to washing, were incubated continuously with the quormone (panels A, B, and C, controls, solid bars) or without the quormone (panels A, B, and C, controls, hatched bars) for the full duration of the experiment. Samples from each of these cultures were assayed for β-galactosidase activity. The experiments shown in panels A and B were repeated twice, and the experiment shown in panel C was repeated three times. In each case, data from a representative repetition are presented.