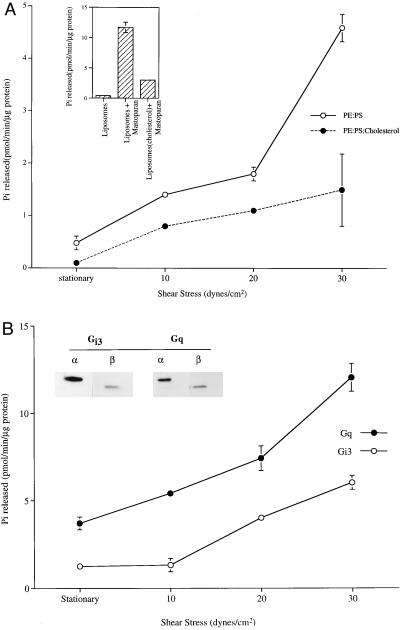
Figure 1.
Shear stress-stimulated GTPase activity of reconstituted G proteins. (A) G protein reconstituted liposomes loaded with [γ-32P]GTP were passed through Sephadex G-50 to remove external GTP. GTPase was stimulated when vesicles were subjected to shear stress (0–30 dynes/cm2) in a cone-and-plate viscometer (37°C, 1 min). This activity was attenuated by incorporation of cholesterol (24 mol %). (Inset) Mastoparan (900 μM) incubated with liposomes for 1 min (37°C) stimulated GTPase activity. (B) Affinity-purified Gαq and Gαi3, reconstituted into liposomes along with their respective βγ subunits (immunoblots for α and β are shown in Inset) were stimulated by shear stress. The values presented here are a mean ± SD of three measurements.