Introduction
Although abnormalities of the anterior cingulate cortex (ACC) have been implicated in a number of psychiatric disorders, including schizophrenia, obsessive–compulsive disorder, depression, post-traumatic stress disorder and autism,1,2,3 it is still unclear how these abnormalities relate to the diversity of cognitive, emotional and behavioural disturbances seen in these disorders. Various authors have put forward models of ACC function4,5 that assume a single overarching role for the region, models which we term “unitary” models. Although these models are based on strong empirical evidence, they are nevertheless incomplete accounts of ACC involvement in cognitive, affective and motor processes. This has led to the interpretation of results without due consideration of the accumulating evidence to suggest that there are at least 3 major functional subdivisions of the ACC.1,6,7 In this paper, we argue that future investigations and interpretations of ACC function in psychiatric research need to take better account of the structural and functional heterogeneity of this region.1,6,7,8,9 This approach may help develop a more comprehensive theory of ACC function to explain the various patterns of symptoms, cognitive and emotional deficits, as well as neuroimaging findings, across a number of psychiatric disorders. We also make suggestions for the design and interpretation of future studies concerning the ACC in psychiatric disorders that may provide important new insights into the neurobiological basis of these disorders.
Anterior cingulate function
The ACC is far from being a functionally homogenous region. Although Broca10 initially emphasized it as a chief component of the limbic lobe (an area where internal drives and emotions are integrated with higher cognitive functions), recent research implicates the ACC as a pivotal component of circuits that also underlie functions such as attention, learning, language and motor behaviour.1,3 It is generally agreed that there are at least 3 major functional subdivisions of the ACC (i.e., affective, cognitive and motor components1,6,7), each of which has different patterns of underlying connectivity and cytoarchitectonic properties (Fig. 1).1,3,7 Such details are particularly relevant when considering the precise localization and clinical correlates of neuropathological changes and suggest that the ACC is likely involved in many aspects of human behaviour, rather than having a specific role.1,3,7
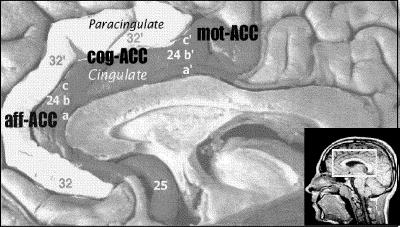
Fig. 1: Anatomical, cytoarchitectonic and functional properties of the anterior cingulate cortex. It is typically comprised of 2 tiers (cingulate and paracingulate) with 3 functional subdivisions: (i) A rostral affective/visceral region (aff-ACC), located inferior and anterior to the genu of the callosum (Brodmann's Areas [BA] 25, 24a-b, 32), which has extensive reciprocal connections with the orbito-frontal cortex and amygdala. This region regulates autonomic and endocrine functions and is also involved in higher-order functions, such as conditioned emotional learning, assessments of motivational content and assigning emotional valence to internal and external stimuli; (ii) A dorsal cognitive region (cog-ACC) lying superior to the callosum (BA 24a'-b', 32'), which has extensive reciprocal connections with other frontal and temporal areas, especially the dorsolateral prefrontal cortex and hippocampus. This region is primarily involved with response selection and cognitively demanding information processing; (iii) A caudal motor region (mot-ACC) deep within the cingulate sulcus (BA 24c', 24c'g) has extensive connections with the primary/supplementary motor and parietal regions and plays a role in premotor/skeletomotor functions (adapted from Mega and Cummings, 1997).27
The limbic and paralimbic anterior cingulate cortex
Based on the cytoarchitecture and connectivity of the ACC, one of the major functional distinctions is between the ventral limbic-ACC (cingulate proper) and the dorsal paralimbic-ACC (paracingulate).7,11 In fact, in 30%–60% of individuals, these 2 functional regions are roughly separated by the paracingulate sulcus.11,12 In these cases, the paracingulate forms a separate gyrus on the medial surface of the brain (Fig. 1), as opposed to being buried in the walls of the cingulate sulcus. However, despite this dorsoventral functional segregation, unitary models of ACC function4,5 tend to describe just 1 ACC function that is particularly relevant to the paracingulate. For example, in a recent article, Sanders and colleagues5 selectively reviewed and provided a model of ACC dysfunction to explain the cognitive deficits seen in schizophrenia. Using evidence of task performance after cingulotomy in a patient with obsessive–compulsive disorder (OCD),13 as well as neuroimaging studies utilizing theory of mind tasks,14 these authors hypothesize that the role of the ACC is to monitor signal mismatches between behaviour and goals. Further, they propose that a disturbance of the ability to monitor signal mismatches between behaviour and goals may reflect certain symptoms seen in schizophrenia such as disorganized language and social and existential dysfunction. However, the 2 lines of evidence used to support this model (i.e., ACC function during theory of mind tasks and ACC dysfunction after cingulotomy) are both associated with the functional integrity of the paracingulate.13,14,15,16
On the basis of a comprehensive series of functional neuroimaging studies, Carter and colleagues4,8 have put forward a similar but more detailed notion of ACC function. They propose that during normal cognition, the ACC serves a specific evaluative function, detecting response competition or “conflict” and indicating the need to implement strategic processes to reduce the conflict and maintain performance. Although Carter and colleagues do not distinguish between the subregions of the ACC that are activated during their tasks, the foci of activation tend to be in the depths of the cingulate sulcus or on the surface of the paracingulate gyrus (i.e., are paralimbic). We argue that this model of ACC function is also primarily relevant to the paracingulate. As such, although our understanding of the functional role of the paracingulate has increased significantly over the past decade, the specific role of the cingulate proper is yet to be elucidated, and this will only be possible if conscious attempts are made to focus on this subregion (i.e., where possible, discriminating between activations in the cingulate and those in the paracingulate, as well as using/designing tasks to specifically probe the cingulate proper).
The affective, cognitive and motor influences of the anterior cingulate cortex
Both Bush et al6 and Picard and Strick17 have collated the findings of a number of functional imaging studies and shown that separate subregions of the ACC are involved in emotion (rostral-ACC) and in cognition and motor control (dorsal- and caudal-ACC, respectively). These important studies provide an anatomical framework for the study of ACC function and are another indication that unitary models of ACC function are not sufficiently complex to explain the involvement of this region in many aspects of human behaviour or how they relate to the behavioural disturbances and symptoms seen across a number of psychiatric disorders. For example, functional neuroimaging studies consistently show that schizophrenia is associated with decreased ACC activation18,19 and that OCD is associated with increased ACC activation.2,20,21 If the ACC merely monitors signal mismatches between behaviour and goals (as suggested by Sanders and colleagues), then it is possible that these findings reflect the under- and over-expression of the same cognitive process such as attention (e.g., decreased attentional responsiveness [schizophrenia] v. increased attentional responsiveness [OCD] to mismatches between behaviour and goals). However, given the functional heterogeneity of the ACC, it is also possible that the findings reflect different cognitive processes, such as attention and emotion, operating in different directions (e.g., decreased emotional responsiveness [schizophrenia] v. increased attentional responsiveness [OCD] to mismatches between behaviour and goals). Alternatively, the symptoms and dysfunction seen in schizophrenia and OCD may be due to dysfunction in a number of subregions of the ACC. For example, schizophrenia may be associated with a decreased input of information to both the affective and cognitive subregions of the ACC, leading to a lack of integration between the individual's attentional and affective or motivational state. Such dysfunction may result in disturbed or reduced goal-directed behaviour and manifest in the form of negative symptoms (e.g., apathy, avolition, hypoarousal and anhedonia). In contrast, OCD may be associated with increased input into these ACC subregions, leading to excessive goal-directed behaviours, reflected as uncontrollable thoughts and anticipatory anxiety. To start addressing these issues, one needs to carefully examine the precise location of ACC dysfunction (i.e., rostral v. dorsal v. caudal and limbic v. paralimbic), because this is likely to indicate whether the dysfunction reflects a cognitive or affective process as well as which interconnected regions may also be involved in driving the dysfunction.
As illustrated in Fig. 1, and recently reviewed by Koski et al22 and Bush et al,6 the functional characteristics of the ACC subdivisions and their effective and functional connections with other brain regions are beginning to be defined. This information can be used in the context of other relevant information about a disorder to guide interpretations about abnormalities in activation patterns associated with these subdivisions.6,7,19,23
Future directions
Considering the subregion, direction, side, nature and extent of dysfunction
That the ACC has been implicated in a number of psychiatric conditions involving a diversity of cognitive and emotional disturbances suggests that it is a vulnerable part of an important and common pathway involved in the cognitive and emotional regulation of behaviour. This may suggest that ACC dysfunction has a nonspecific association with psychopathology, but it is also possible that the same region is involved in a different manner in different disorders. For example, the nature of the symptoms or behavioural disturbances seen in various psychiatric disorders may be dependent on the subregion implicated (i.e., rostral v. dorsal v. caudal; limbic v. paralimbic), as well as the direction (e.g., increased, decreased, disorganized), side (i.e., left, right, bilateral), nature (e.g., cellular, neurochemical, structural, connectivity, metabolic) and extent (e.g., single v. multiple subregions, side and nature) of abnormality. The number of possible combinations suggests that different patterns of abnormalities within the ACC may indeed be associated with the different behavioural disturbances and symptoms seen in psychiatric disorders. Whether this is the case will be determined on the basis of future studies that give due consideration to these issues.
An integrated approach and considering the individual within the group
Until recently, most neuroimaging studies used a single or 2-dimensional approach to psychiatric research (e.g., functional imaging measures in isolation or combined with structural measures) and conducted group-averaged (pooled data) analyses. These studies are useful, but many related questions are often left unanswered (e.g., whether ACC hypoactivation is due to underlying structural anomalies, such as reduced volume, disturbed connectivity, neurochemical anomalies, or to interindividual variability or artifact). We propose that studies utilizing multiple but complimentary neuroimaging modalities concerning the micro- (magnetic resonance spectroscopy, diffusion tensor imaging) and macrostructural (magnetic resonance imaging [MRI]) properties of the ACC, together with dynamic (functional-MRI and electrophysiological) data assessing the same region are now required to advance this field of research. Most importantly, these investigations should be performed concurrently in the same individual to minimize the confounds of interindividual differences but, at the same time, allow for comprehensive analyses of individual data. Such multimodal within-subject studies are now feasible with recent developments in MRI technology and tasks designed to probe specific subregions of the ACC.24,25,26 This approach will enable us to definitively answer questions concerning the relation between different patterns of ACC dysfunction and how they might relate to the diversity of symptoms and behavioural disturbances seen in various psychiatric disorders and thereby provide important new insights into the neurobiological basis of psychiatric disorders.
Conclusions
There is now extensive evidence from the neuroimaging literature linking ACC dysfunction to a number of psychiatric disorders, including schizophrenia, OCD, depression, bipolar disorder, post-traumatic stress disorder and autism. Given the involvement of the ACC in many cognitive and affective processes, this is not surprising and suggests that the ACC is a vulnerable part of an important and common pathway involved in the cognitive and emotional regulation of behaviour. However, models of ACC function are incomplete, and precisely how ACC dysfunction is related to the diversity of symptoms and behavioural disturbances seen in psychiatric disorders is still unclear. To achieve a better understanding of ACC function (and dysfunction), we must consider the structural and functional heterogeneity of the ACC when designing studies and interpreting findings related to this structure. Further, we need to expand and test the initial cognitive and neurobiological theories of these psychiatric disorders using integrated multimodal neuroimaging approaches and tasks designed specifically with ACC heterogeneity in mind. By doing so, and giving consideration to the individual within the group, we can more precisely localize the regions of ACC dysfunction and better identify their clinical correlates, and thereby understand their implications for various psychiatric disorders, and potentially, the individual.
Acknowledgments
This research was also supported by the National Health and Medical Research Council (NHMRC ID: 970599; 236175) and the NHMRC Brain Research Network, Woods Family and the Ian Potter Foundation, Melbourne, Australia.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Murat Yücel, Cognitive Neuropsychiatry Unit, c/o Mental Health Research Institute, Locked Bag 11, Parkville, Victoria, Australia 3052; fax 61 (3) 9387 5061; murat.yucel@wh.org.au.
Submitted Nov. 26, 2002 Revised Feb. 11, 2003 Accepted Mar. 18, 2003
References
- 1.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118:279-306. [DOI] [PubMed]
- 2.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994;51:62-70. [DOI] [PubMed]
- 3.Benes FM. Relationship of cingulate cortex to schizophrenia and other psychiatric disorders. In: Vogt BA, Gabriel MS, editor. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook. Boston: Birkhauser; 1993. p. 580-605.
- 4.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 1999;10:49-57. [DOI] [PubMed]
- 5.Sanders GS, Gallup GG, Heinsen H, Hof PR, Schmitz C. Cognitive deficits, schizophrenia, and the anterior cingulate cortex. Trends Cogn Sci 2002;6:190-2. [DOI] [PubMed]
- 6.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4:215-22. [DOI] [PubMed]
- 7.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001;2:417-24. [DOI] [PubMed]
- 8.Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A 2000;97:1944-8. [DOI] [PMC free article] [PubMed]
- 9.Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 1995;359:490-506. [DOI] [PubMed]
- 10.Broca P. Anatomie compare des circonvolutions cerebrales: le grande lobe limbique. Rev Anthropol 1878;1:385-498.
- 11.Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, et al. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex 1996; 6:207-14. [DOI] [PubMed]
- 12.Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb Cortex 2001;11:17-25. [DOI] [PubMed]
- 13.Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, et al. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia 2001;39:219-30. [DOI] [PubMed]
- 14.Frith CD, Frith U. The biological basis of social interaction. Curr Dir Psychol Sci 2001;10:151-5.
- 15.Frith CD, Frith U. Interacting minds—a biological basis. Science 1999;286:1692-5. [DOI] [PubMed]
- 16.Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 2000;38:11-21. [DOI] [PubMed]
- 17.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996; 6: 342-53. [DOI] [PubMed]
- 18.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997;154:1670-5. [DOI] [PubMed]
- 19.Yücel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, et al. Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry 2002;159:251-4. [DOI] [PubMed]
- 20.Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry 2001;50:659-67. [DOI] [PubMed]
- 21.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 2000;34:317-24. [DOI] [PubMed]
- 22.Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res 2000;133:55-65. [DOI] [PubMed]
- 23.Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 1998;9:37-47. [DOI] [PubMed]
- 24.Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry 2003;8:60-70. [DOI] [PubMed]
- 25.Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 1998;6:270-82. [DOI] [PMC free article] [PubMed]
- 26.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 1998;44:1219-28. [DOI] [PubMed]
- 27.Mega MS, Cummings JL. The cingulate and cingulate syndromes. In: Cummings JL, Trimble MR, editors. Contemporary behavioral neurology. Boston: Butterworth Heinemann; 1997. p. 189-214.


