Figure 1.
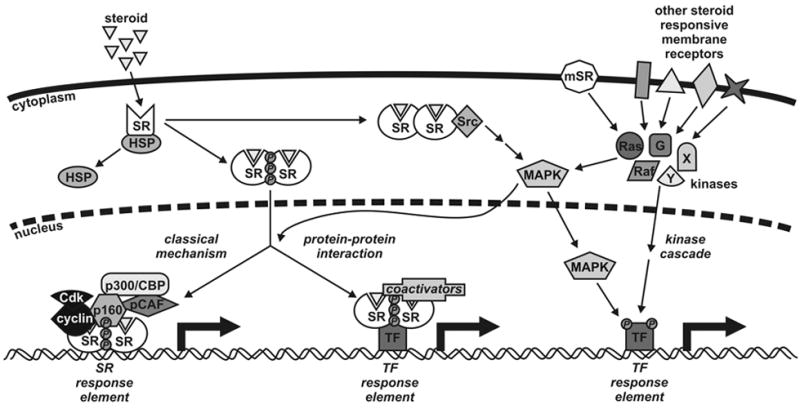
Model for steroid receptor (SR) action. Steroid hormones difuse across the cell membrane where they bind to their cognate receptor in the cytoplasm of target cells. Ligand binding induces conformational changes in the receptor, dissociation of heat shock proteins (HSPs), dimerization and nuclear translocation. In the classical mechanism for SR action, the SR dimer binds to specific DNA response elements situated in the regulatory regions of target genes. This is followed by recruitment of coactivators, such as p160s, cyclins, p300/CBP and pCAF, and other components of the general transcription machinery enabling RNA synthesis. SRs also influence transcription regulated by other transcription factors (TFs) through protein-protein interactions and coactivator recruitment rather than DNA binding. These mechanisms are in turn influenced by phosphorylation of the receptor by multiple kinase pathways such as Src, MAPK, Ras, Raf and G-proteins. Both cytoplasmic and membrane bound receptors (mSRs) influence kinase signaling cascades leading to the phosphorylation of transcription factors. Other steroid responsive receptors with no homology to nuclear receptors are also capable of altering cellular signaling.
