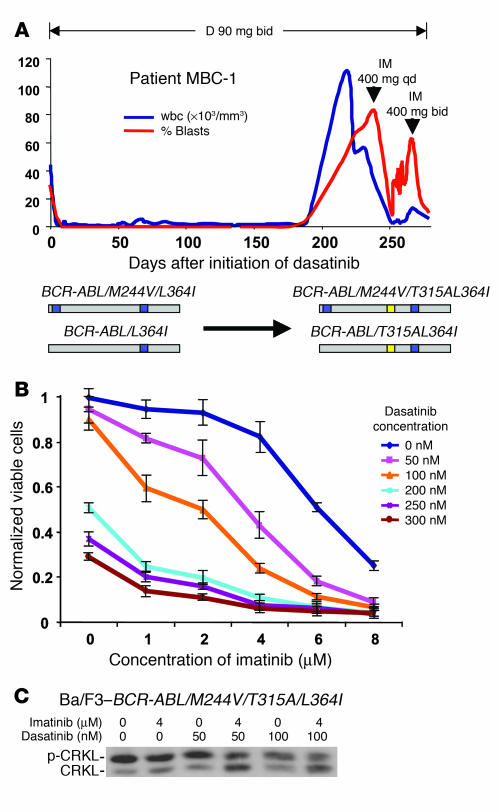
Figure 3. Additive BCR-ABL inhibitory properties of 2 drugs can lead to clinical responses in cases harboring compound mutations resistant to both agents.
(A) wbc count (blue) and peripheral blood myeloblast percentage (red) in MBC-1, who developed resistance associated with the compound mutation M244V/T315A/L364I in BCR-ABL in 22 of 27 clones sequenced, then responded to a combination of dasatinib and imatinib. This patient initially relapsed on imatinib with 2 subclones, 1 with the mutant L364I and a second clone bearing the M244V/L364I compound mutant (both M244V and L364I are known imatinib-resistant mutants). (B) Dasatinib and imatinib cooperatively inhibit the growth of Ba/F3 cells harboring BCR-ABL/M244V/T315A/L364I in vitro. Combination therapy had no effect on parental IL-3–dependent Ba/F3 cells (data not shown). (C) CRKL Western immunoblot of Ba/F3–BCR-ABL/V299L exposed to clinically relevant concentrations of imatinib and dasatinib.