Figure 8.
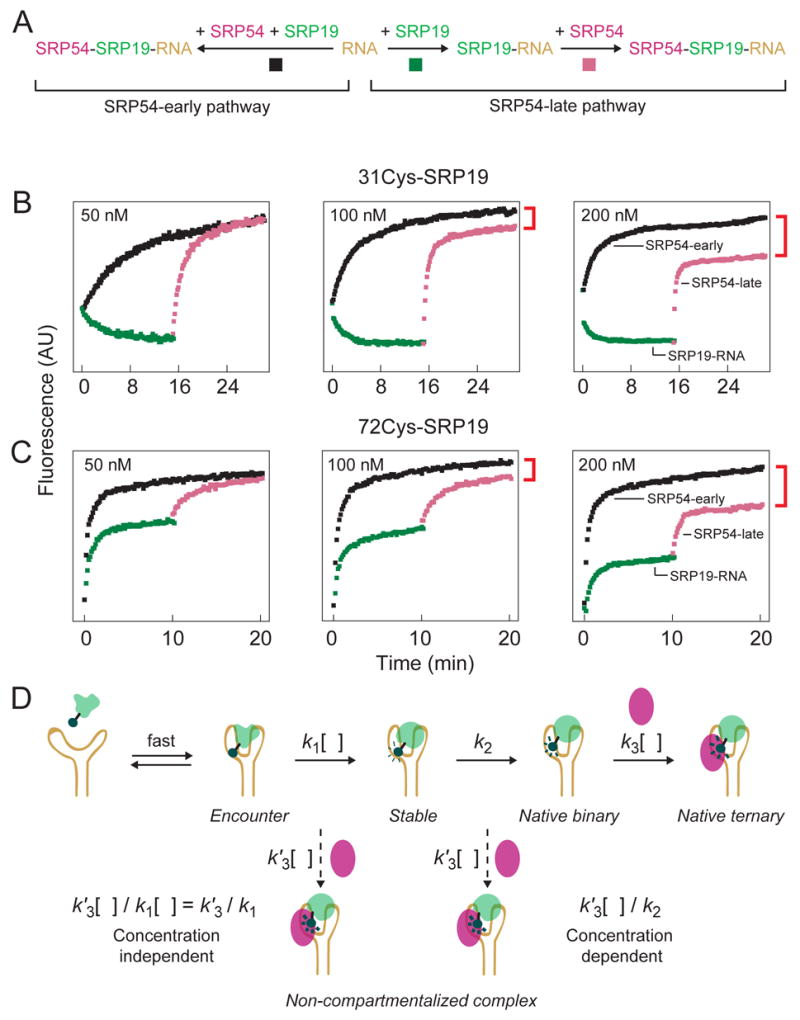
The extent of non-compartmentalized complex formation is concentration dependent. (A) Scheme for monitoring native vs. non-compartmentalized assembly using single fluorophore experiments. Native assembly (the SRP54-late pathway) involves ordered binding by SRP19 (green traces) and SRP54 (purple traces). For non-compartmentalized assembly (the SRP54-early pathway), both proteins assemble simultaneously (black traces). (B,C) Visualization of native and non-compartmentalized ternary complex formation using single fluorophore experiments. Ratios of SRP19, SRP54 and RNA components were held constant at 1:1:2 in all experiments; protein concentrations are given for each panel. Non-compartmentalized complex formation is reported directly as the intensity difference between purple and black traces (see red brackets). (D) Expected concentration dependence for SRP54 binding post-Encounter versus post-Stable complex formation. k1 is a compound rate constant reflecting formation of the Stable complex via the kinetically linked Encounter complex. Dashed arrows show putative, concentration-dependent (k′3[ ]), steps for SRP54-mediated misassembly.
