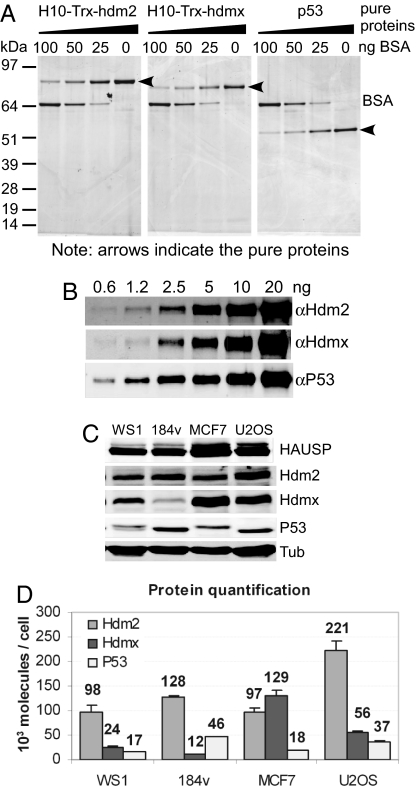
Fig. 1.
Protein quantification using LiCor and Western blotting. (A) Serially diluted pure recombinant proteins were applied to each lane of a gradient polyacrylamide gel along with different known concentrations of BSA as a standard. The gel was stained with SYPRO-ruby, and the intensities of protein staining were measured by using the Typhoon image system. (B) The linearity of the system was determined by Western blotting of serially diluted known concentrations of pure recombinant P53, Hdm2, and Hdmx. Signals were analyzed by using the LiCor system. (C) Protein analyses in equal numbers of WS1, 184V, MCF7, and U2OS cells. Lysates obtained from the same numbers of cells were run on an 8% acrylamide gel along with a mix of serially diluted protein standards (data not shown) and immunoblotted with antibodies against HAUSP, Hdm2, Hdmx, P53, and α-tubulin. (D) Protein quantification based on the band intensities from the Western blot in C. All values were derived by using the LiCor system. The amounts of each protein were calculated by using band intensities from a known concentration of the respective pure proteins as standards and with known cell numbers from lysate preparation. Error bars represent SD of three experiments. Numbers represent the protein molecules × 103 per cell.