Figure 4.
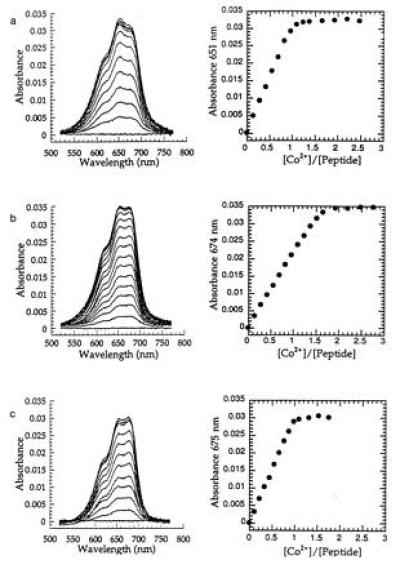
Absorption spectra of Co2+ complexes of proteins and peak absorbance vs. equivalents of metal bound of Nup475SD (a); Nup475DD (b); and Nup475DD(Y143K) (c). The Nup475SD spectra shown are at 43.9 μmol peptide, the Nup475DD spectra are at 26.2 μmol protein, and the Nup475(Y143K) spectra are at 30.1 μmol protein. The overall shapes of the d-d absorbances are similar to previously reported TFIIIA-type zinc fingers mutated to have Cys3His tetrahedral coordination (17). One of the effects of the Tyr to Lys mutation in Nup475(Y143K) (c) is that the mutant binds only one equivalent of metal, unlike the wild-type protein from which it was derived (b). Zinc back-titrations were performed in the same system using a 300-fold excess of cobalt.
