Abstract
We examined whether mutation of the δ-sarcoglycan gene, which causes dilated cardiomyopathy, also alters the vascular smooth muscle cell (VSMC) phenotype and arterial function in the Syrian hamster CHF 147. Thoracic aorta media thickness showed marked variability in diseased hamsters with zones of atrophy and hypertrophied segments. CHF-147 VSMCs displayed a proliferating/“synthetic” phenotype characterized by the absence of the smooth muscle myosin heavy chain SM2, dystrophin, and Ca2+-handling proteins, and the presence of cyclin D1. In freshly isolated VSMCs from CHF 147 hamsters, voltage-independent basal Ca2+ channels showed enhanced activity similar to that in proliferating wild-type (WT) cells. The transcription factor NFAT (nuclear factor of activated T cells) was spontaneously active in freshly isolated CHF 147 VSMCs, as in proliferating VSMCs from WT hamsters. Mibefradil inhibited B-type channels, NFAT activity, and VSMC proliferation. CHF 147 hamsters had abundant apoptotic cells distributed in patches along the aorta, and clusters of inactive mitochondria were observed in 25% of isolated CHF 147 cells, whereas no such clusters were seen in WT cells. In conclusion, mutation of the δ-sarcoglycan gene increases plasma membrane permeability to Ca2+, activates the Ca2+-regulated transcription factor NFAT, and leads to spontaneous mitochondrial aggregation, causing abnormal VSMC proliferation and apoptosis.
Disruption of the plasma membrane-associated sarcoglycan-sarcospan complex as a result of genetic defects causes muscular dystrophy and/or cardiomyopathy in humans (limb-girdle muscular dystrophy).1 There are six sarcoglycan family members: α-, β-, γ-, δ-, ε-, and ζ-sarcoglycan.2 In hamster and mouse models, δ-sarcoglycan gene deletion results in myopathy of cardiac and skeletal muscles, with focal areas of necrosis3,4,5 and autophagic cardiomyocyte death.6 Most of the studies on δ-sarcoglycan-deficient animals have been conducted on skeletal and cardiac muscles. The few studies on smooth muscle concerned the vasospasm of coronary arteries, but there are no data on the peripheral vessels. Sarcoglycans are transmembrane components of the dystrophin-glycoprotein complex, which links the cytoskeleton to the extracellular matrix.7 At the cellular level, disruption of the dystrophin-glycoprotein complex leads to increased permeability to divalent cations through channel-blocker-sensitive pathways and entry of calcium via nonspecific cation channels.8,9,10,11 The mechanisms of this enhanced Ca2+ influx are not fully understood, but changes in the activity of several Ca2+ channels have been described in dystrophin-deficient myocytes.12,13,14,15 Dystrophin, through PDZ domain-containing adaptor proteins known as syntrophins, can link the cytoskeleton to various membrane proteins carrying a PDZ domain, including ion channels.16 This cytoskeleton-ion channel interaction contributes to receptor/channel localization and to the regulation of voltage-, ligand-, and store-operated ion channels. Indeed, restoration of functional dystrophin-sarcoglycan complex formation by gene transfer of minidystrophin or δ-sarcoglycan normalizes ion channel function in dystrophic myocytes.10,12,17,18
In vascular smooth muscle cells (VSMCs), Ca2+ homeostasis not only controls vessel tone but also defines the cell phenotype (from quiescent/contractile to proliferating/“synthetic”). The proliferating/synthetic phenotype is associated with a reduction in contractile performance owing to the loss of adult isoforms of contractile proteins and dystrophin.19 Moreover, proliferating VSMCs lose RyR and sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) 2a,20 LTCC (L-type Ca2+ channels) are replaced by TTCC (T-type Ca2+ channels), and SOC (store-operated channels) as well as TRPCs (transient receptor potential protein family C) are up-regulated.21 This results in an increased cytosolic Ca2+ concentration and changes in the spatiotemporal pattern of Ca2+ signals, which can alter gene expression by activating various protein kinases and phosphatases and Ca2+-sensitive transcription factors.22,23 For instance, a sustained increase in cytosolic Ca2+ is necessary to activate calcineurin, a Ca2+/calmodulin-dependent serine/threonine-specific protein phosphatase 2B (PP2B) that dephosphorylates nuclear factor of activated T cells (NFAT), inducing its translocation into the nucleus and transcriptional activation. NFAT is involved in the control of cell cycle-related proteins required for VSMC proliferation24,25
The aim of this study was to determine the consequences of δ-sarcoglycan gene mutation on vessels of CHF 147 myopathic Syrian hamsters. We postulated that alterations of the dystrophin/sarcoglycan complex would be associated with enhanced transmembrane Ca2+ influx and with activation of Ca2+-dependent processes in VSMCs.
Materials and Methods
Animals
Animals were treated in accordance with institutional guidelines. The study was performed on thoracic aortas from 6- to 12-month-old male and female cardiomyopathic Syrian hamsters of the strain CHF 147 (raised by INSERM U582, Paris, France) and their control Golden hamsters (WT) obtained from Janvier-France breeders.
Materials
All media, sera, and antibiotics were from Invitrogen (Cergy Pontoise, France). All chemicals were from Sigma-Aldrich (Saint Quentin Fallavier, France). The following primary antibodies were used: anti-SERCA 2a and anti-SERCA 2b (provided by Dr. F. Wuytack, University of Leuven, Leuven, Belgium),26 anti-RyR (provided by I. Marty, INSERM U607, Département Réponse et Dynamique Cellulaires-Grenoble, France),27 anti-SM2 (Ab 683; Abcam plc, Cambridge, UK), anti-NM-MHC-B (Ab 684; Abcam), anti-dystrophin (NCL-DYS2; Novocastra, Newcastle, UK), anti-caveolin 1 (ab2910; Abcam), anti-PMCA (ab2825; Abcam), anti-cyclin D1 (556470; BD Biosciences), and anti-NFATc1 (K-18; Santa Cruz Biotechnology, Santa Cruz, CA).
Histology and Immunofluorescence Studies
Media thickness was measured on hematoxylin and eosin-stained frozen cross sections with a computer-based morphometric system (Lucia; Nikon, Tokyo, Japan). Ten measurements were made on each section, and five discontinuous sections were analyzed in each animal. Ten CHF 147 and 10 WT hamsters were studied.
Apoptosis was analyzed by terminal deoxynucleotidyl transferase dUTP nick-end labeling staining of fixed cross sections with a standard protocol (ApopTag Red; Serologicals Corporation, Norcross, GA). Immunocytochemical analysis was applied to methanol-fixed cells or acetone-fixed sections according to a standard protocol (Santa Cruz Biotechnology). Proteins were visualized by using either secondary antibodies directly conjugated to Texas Red or the biotin/streptavidin-Texas Red-conjugated amplification method (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Nuclei were labeled with Hoescht.
Cell Culture
VSMCs were isolated from the thoracic aorta of Syrian CHF 147 and WT hamsters and cultured as described elsewhere.28 Proliferation was measured by using the CellTiter96 Cell Proliferation Assay kit (Promega, Charbonnières, France).
Single-Channel Recordings and Data Analysis
Experiments were performed with the cell-attached and/or inside-out patch-clamp configuration. Patch pipettes (10 to 15 mol/LΩ) were pulled from borosilicate glass capillaries (Corning Kovar Sealing 7052; WPI, Sarasota, FL). Currents were recorded with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Channel activity (relative mean membrane patch current) was calculated as previously reported.29 All experiments were conducted at room temperature (20 to 24°C). The superfusion control and bath solutions contained 128 mmol/L potassium aspartate, 2 mmol/L KCl, 1 mmol/L BaCl2, 5 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 10 mmol/L HEPES, and 10 mmol/L glucose; pH was adjusted to 7.4 with KOH. The pipette solution contained 48 mmol/L BaCl2 and 10 mmol/L HEPES; pH was adjusted to 7.4 with CsOH. When the patch pipette contained 48 mmol/L Ba2+, and the patch membrane potential was continuously held at −80 mV (no voltage pulses being applied), spontaneous inward currents corresponding to channel opening were recorded. Ba2+ was used as charge carrier because it is generally considered that Ba2+ ions are more permeable than Ca2+ ions through several types of Ca2+ channels (L- and T-type), and this property seems to hold for B-type Ca2+ channels.30 Because Ba2+ blocks practically all known K+, Na+, and Cl− channels, it is useful for studying Ca2+ channels. We also wanted to avoid activating Ca2+-activated K+ or Cl− channels by Ca2+ flowing through these channels. We used blockade by eosin applied to the internal face of the membrane patch to confirm the presence of B-type channels.
Confocal Microscopy
Slides were examined with a Zeiss LSM-510 laser scanning confocal microscope (Carl Zeiss GmbH, Jena, Germany) equipped with a 30-mW argon laser and a 1-mW helium-neon laser, using a 20X/NA 0.75 Plan-Apochromat or 63X/NA 1.40 Plan Apochromat oil immersion objective. Green fluorescence was observed with a 505- to 550-nm band-pass emission filter under 488-nm laser illumination. Red fluorescence was observed with a 560-nm long-pass emission filter under 543-nm laser illumination. Pinholes were set at 1.0 Airy unit. Stacks of images were collected every 0.9 μm along the z axis. All settings were kept constant for reasons of comparability. Three median slices were projected for NFAT samples. For double immunofluorescence, dual excitation using the multitrack mode (images acquired sequentially) was obtained with the argon and He/Ne lasers.
Promoter Reporter Assay
Cells were transfected using FuGene 6 (Roche, Basel, Switzerland) with NFAT-promoter-luciferase construct (NFAT-Luc; Stratagene, Cambridge, UK). They were cultured for 48 hours without fetal calf serum (FCS) and then treated with 10% FCS alone or together with 5 μmol/L mibefradil or 10 μmol/L cyclosporine A (CsA) for 5 hours. The luciferase activity was measured by using “the luciferase assay kit” (Promega). It was expressed as percentage of control in relative luciferase units.
Mitochondrial Architecture and Activity
For live cell confocal microscopy, cells were plated on a collagen-coated coverglass chamber (Lab-Tek II Chamber 1.5 German Cover Glass System; Nalge Nunc International, Rochester, NY) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FCS for 24 to 48 hours. Then, the medium was replaced by Ham’s F-12, with 25 mmol/L HEPES, without phenol red (Promocell, Heidelberg, Germany). To assess changes in mitochondrial membrane (Δm potential), live cells were double-labeled with the mitochondrion-sensitive Δm-independent probe MitoTracker Green (500 nmol/L, M7514; Invitrogen, Carlsbad, CA) and the Δm-sensitive dye MitoFluor Red (500 nmol/L, M22422; Invitrogen) according to manufacturers’ instructions. Cells were observed in an inverted confocal microscope equipped with a chamber at 37°C. Fluorescence was recorded by means of confocal laser scanning microscopy (Leica TCS4D; Wetzlar, Germany) (λex, 490 and 598 nm; λem, 516 and 630 nm, respectively).
Statistical Analysis
All quantitative data are means ± SEM of at least three independent experiments. An unpaired t-test was used to compare means. The nonparametric Mann-Whitney test was used to compare media thickness. Differences were considered significant when P < 0.05.
Results
Altered Phenotype of CHF 147 Thoracic Aorta VSMCs
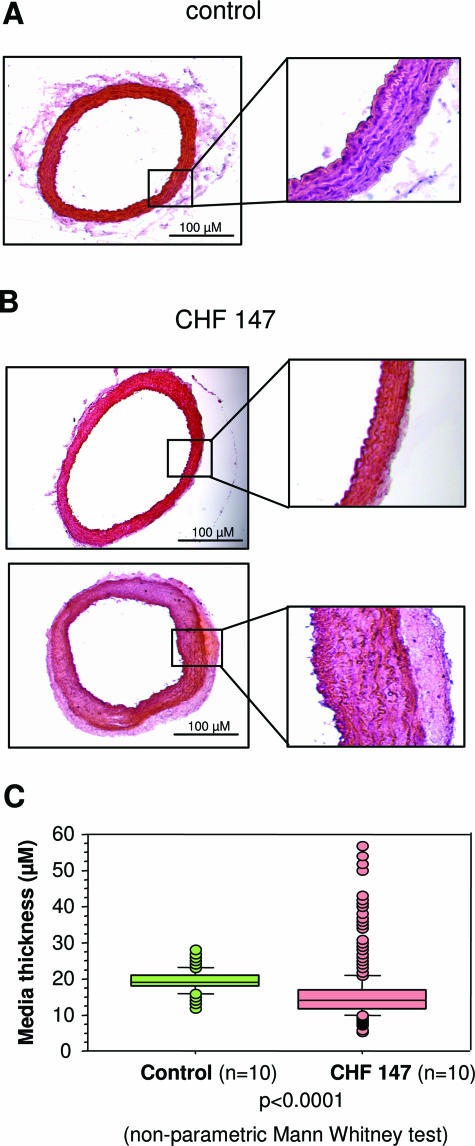
In WT hamsters, media thickness was homogenous (Figure 1, A and C), whereas in CHF 147 hamsters, there were numerous zones of atrophy and marked hypertrophy (Figure 1B). One CHF 147 animal had global hypertrophy of the media, two had aspects similar to WT, and seven were globally atrophic. The mean CHF 147 value was lower than the WT value (15.8 ± 6.9 versus 19.4 ± 2.8 μm; P < 0.001).
Figure 1.
Morphometric analysis of the thoracic aorta from WT and cardiomyopathic (CHF 147) hamsters. Histological cross sections from WT (A) and CHF 147 hamsters (B) stained by hematoxylin-eosin are shown. The right panels are magnifications of the aortic wall. C: Distribution of media thickness.
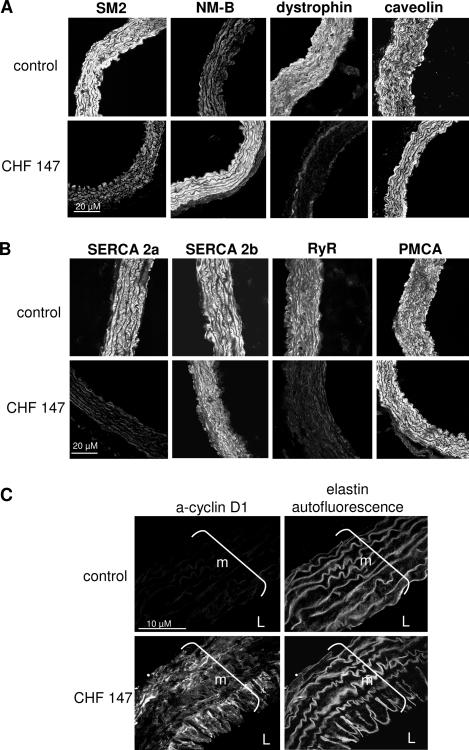
To characterize the phenotype of CHF 147 VSMCs, aorta sections from six WT and six CHF 147 animals were labeled with antibodies specific for the contractile or synthetic phenotype and were then examined by immunofluorescence (Figure 2). The adult smooth muscle myosin heavy chain SM2 was present in aortic VSMCs from WT hamsters but was extensively replaced by the nonmuscular myosin heavy chain NM-B, a marker of the synthetic phenotype, in CHF 147 hamsters. Dystrophin, another marker of the contractile phenotype, was present in WT and absent in CHF 147 aortas, whereas caveolin-1, a specific marker of membrane caveolae, was expressed in both WT and myopathic animals.
Figure 2.
Phenotype of smooth muscle cells from thoracic aorta. Confocal immunofluorescence. A: Expression of markers of the synthetic phenotype: nonmuscular myosin heavy chain B (NM-B) and the contractile phenotype: smooth muscle myosin heavy chain 2 (SM2) and dystrophin. Expression of caveolin 1 is also shown. B: Expression of calcium-handling proteins: SERCA 2a, SERCA 2b, RyR, and PMCA. C: Expression of cyclin D1, a marker of cell proliferation. m, media; L, lumen.
Both SERCA 2a and RyR were present in the media of WT hamsters but absent from CHF 147 animals, as previously shown in proliferating VSMCs.20,28 The other SERCA isoform, SERCA 2b, and the plasma membrane Ca2+ pump PMCA were present in both WT and CHF 147 animals. This suggested that CHF 147 VSMCs had a synthetic phenotype. Labeling with anti-cyclin D1, a marker of proliferation, confirmed the presence of proliferating cells in the media, and especially in the luminal part of the aorta, in five out of six CHF 147 animals studied; WT aortas were negative for cyclin D1 staining (Figure 2C). Cyclin D1 expression was variable along the aorta, with zones of strong expression and other zones of no expression. Thus, the CHF aorta displayed a heterogeneous pattern of undifferentiated/proliferating VSMCs. The apparent discrepancy between the proliferative phenotype of the VSMCs and the atrophic phenotype of the vessel might be due to apoptosis in the vessel wall (see VSMCs from CHF 147 Undergo Apoptosis).
Proliferative Properties of Isolated VSMCs
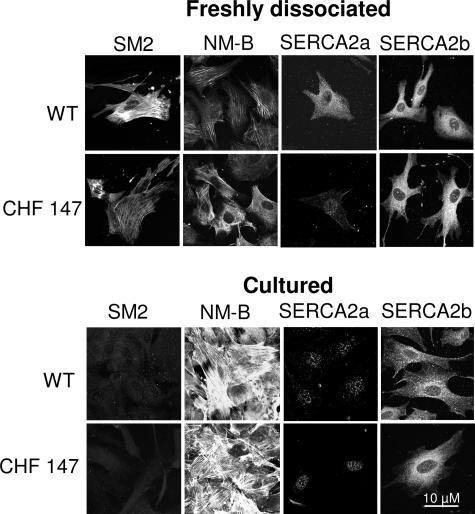
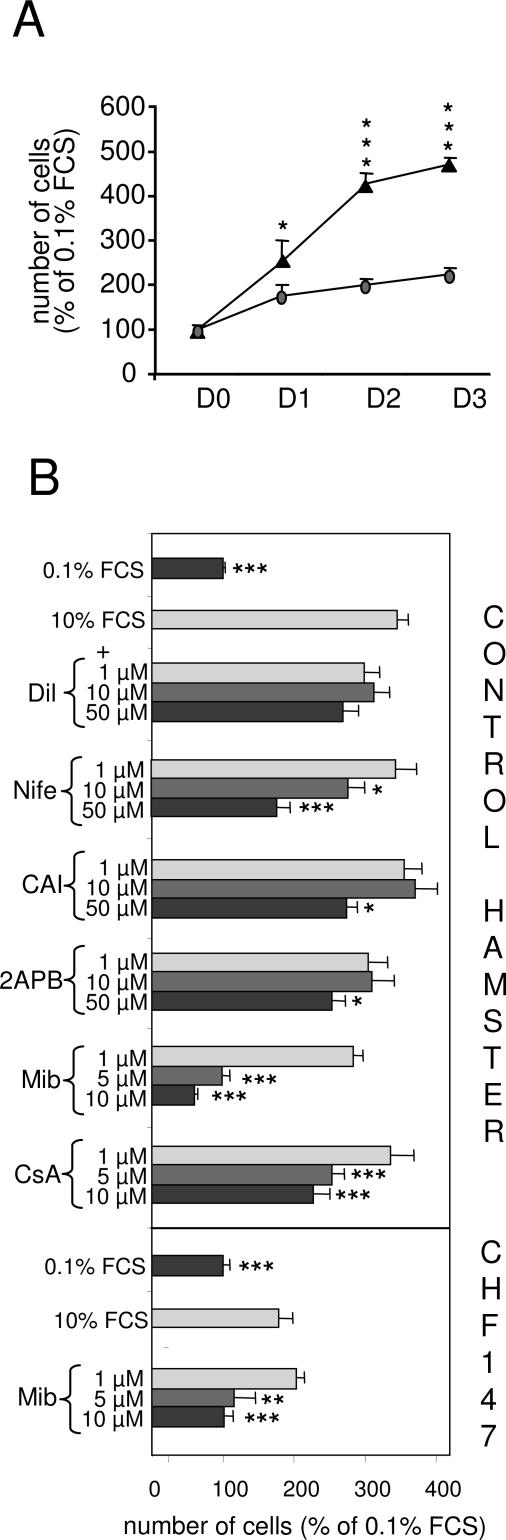
Freshly isolated VSMCs from WT hamsters expressed markers of the differentiated phenotype, such as SM2 and SERCA 2a, whereas these markers were absent from freshly isolated VSMCs from CHF 147 hamsters while NM-B MHC was present. Freshly dissociated VSMCs from CHF 147 hamsters resembled proliferating VSMCs from WT hamsters (Figure 3). When stimulated with serum (10%), the number of VSMCs in WT increased 4.5-fold after 2 days in culture, compared with less than twofold with VSMCs from CHF 147 hamsters cultured in the same conditions (Figure 4A).
Figure 3.
Characterization of primary VSMCs. A: Analysis of the phenotype of freshly dissociated or cultured (3 days, 10% FCS) VSMCs. Confocal immunofluorescence with specific antibodies to smooth muscle myosin heavy chain 2 (SM2), to the nonmuscle myosin heavy chain-B (NM-B), and to SERCA 2a and SERCA 2b is shown.
Figure 4.
Proliferation of primary VSMCs in culture. A: Analysis of the growth capacity of VSMCs from WT (▴) and CHF 147 (•) in culture. Freshly isolated cells were cultured in the presence of serum (0.1 or 10%) for 24 to 72 hours. Each point represents the mean of three to five independent experiments. At each time point the number of cells was normalized to the number of cells in 0.1% FCS (control). Values are plotted as a percentage of the control value. *P < 0.05, ***P < 0.01 WT versus CHF 147 at the same time point. B: Pharmacological analysis of the calcium signaling pathways of serum-induced proliferation. VSMCs (passages 1 to 3) were cultured for 48 hours in the presence of 0.1 or 10% FCS, either alone (control) or together with drugs at the indicated concentrations. Dil, diltiazem; Nife, nifedipine; CAI, carboxyamidotriazole; 2APB, 2-aminoethoxydiphenyl borate; Mib, mibefradil. Data are expressed as a percentage of the cell number in control wells. The results represent the average of at least four independent experiments performed in tetraplicate. **P < 0.05, ***P < 0.001 versus control.
Effect of Ca2+ Inhibitors on Proliferation
Increased plasma membrane permeability to Ca2+ has been described in cardiac and skeletal myocytes lacking δ-sarcoglycan.8,9,10,11 In VSMCs, elevated cytosolic Ca2+ levels induce proliferation and phenotypic changes.21 We therefore tried to detect enhanced Ca2+ entry in CHF 147 VSMCs by using various Ca2+ inhibitors (Figure 4B). Diltiazem, an inhibitor of LTCC, had no effect on the proliferation of VSMCs from WT hamsters. Nifedipine, another LTCC inhibitor, prevented WT VSMC proliferation but only at a high concentration. Mibefradil, a putative T-type Ca2+ channel blocker, completely blocked VSMC proliferation when used at a low concentration (5 μmol/L). Carboxyamidotriazole and 2-aminoethoxydiphenyl borate, two nonspecific inhibitors of capacitative calcium entry, inhibited proliferation only partially and only at high concentrations. Because mibefradil most efficiently inhibited the proliferation of WT hamster VSMCs, we also tested its effect on serum-induced proliferation of CHF 147 VSMCs. As in WT, 5 μmol/L mibefradil completely inhibited the increase in cell numbers induced by serum. Because of the small amount of cells obtained from mutant hamsters, we were unable to test the other inhibitors. These results indicate that Ca2+ entry is involved in VSMC proliferation. The calcineurin inhibitor CsA partially blocked the proliferation of VSMCs from WT hamsters (Figure 4B), suggesting the involvement of the calcium-dependent calcineurin/NFAT-signaling pathway in the proliferation of hamster VSMCs.
Analysis of B-Type Channels in VSMCs from Control and CHF 147 Hamsters: Effect of Mibefradil
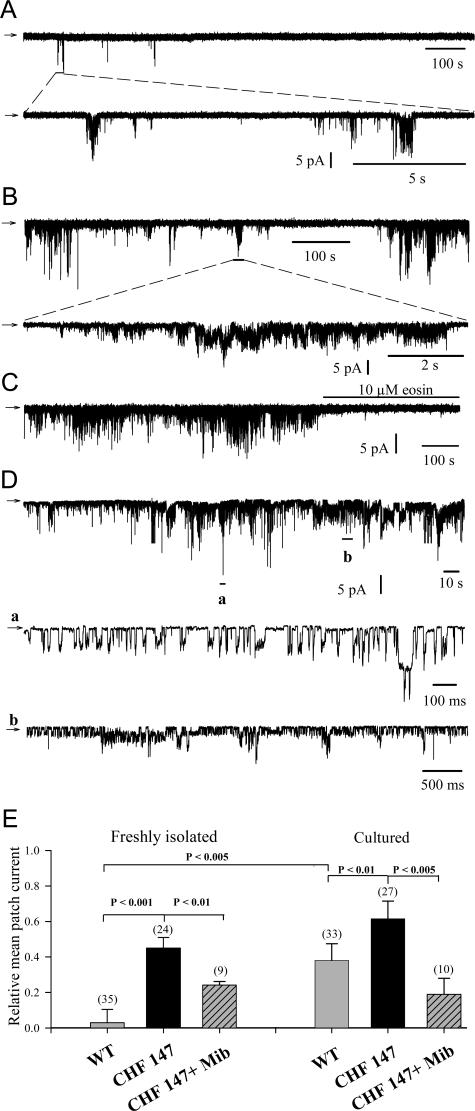
To examine further the possibility of enhanced Ca2+ entry in CHF 147 VSMCs, we performed cell-attached and inside-out patch recordings using membranes of VSMCs freshly isolated from WT (Figure 5A) and CHF 147 hamsters (Figure 5B). In WT cells, single-channel activity (observed in 15% of membrane patches tested) exhibited rare bursts of intense activity followed by long-lasting quiescent periods. Channel activity, assessed as the relative mean patch current, was 0.030 ± 0.075 (mean ± SD, n = 35) (Figure 5E) in WT VSMCs. In contrast, in freshly isolated VSMCs from CHF 147 hamsters, spontaneous channel activity (35% of tested membrane patches) exhibited longer bursts of intense activity, separated by shorter quiescent periods (Figure 5B). The relative mean patch current was markedly enhanced to 0.45 ± 0.06 (n = 24) (Figure 5E) and was very similar to that observed in proliferating WT VSMCs (0.38 ± 0.09). Cultured CHF 147 VSMCs also showed marked channel activity (0.615 ± 0.10, n = 27) (Figure 5, D and E). The expanded-time scale trace designated “a” in Figure 5D shows the complex gating pattern of B-type channels, which was very similar to that previously observed with cardiac myocytes when B-type channel activity was induced by chlorpromazine.31 Eosin (10 μmol/L) completely blocked the channel activity (Figure 5C), an effect characteristic of B-type Ca2+ channels.31 When channel recordings were made with mibefradil (20 μmol/L) in the pipette, channel activities in freshly isolated and cultured CHF 147 VSMCs were markedly reduced (to 0.24 ± 0.02 and 0.19 ± 0.02, respectively; Figure 5E).
Figure 5.
B-type Ca2+ channel activity in primary VSMCs. B-type Ca2+ channel activity detected in membrane patches of freshly isolated WT VSMCs (A), freshly isolated CHF 147 VSMCs (B), cultured WT VSMCs (C), and cultured CHF 147 VSMCs (D). E: Bar graphs compare the relative mean patch current in experiments conducted as shown in A–D. The top traces are representative cell-attached recordings of spontaneous bursts of activity, showing the sporadic bursting nature of this channel activity obtained during application of control superfusion to the cell. The bottom traces are expanded time-scale extracts, as indicated. Arrows indicate zero current (closed level), and the downward deflexions are inwardly directed membrane currents. In D, bottom traces noted a and b are expanded time-scale extracts, as indicated. Numbers and bars denote the numbers of measurements obtained with different patches and corresponding SD value, respectively. P value was determined with Student’s t-test. The membrane patch holding potential was −80 mV.
Thus, freshly isolated VSMCs from CHF 147 possess voltage-independent basal Ca2+ channels with enhanced activity similar to that of proliferating WT cells. Mibefradil inhibited the activity of these channels.
NFAT Is Activated in CHF 147 VSMCs
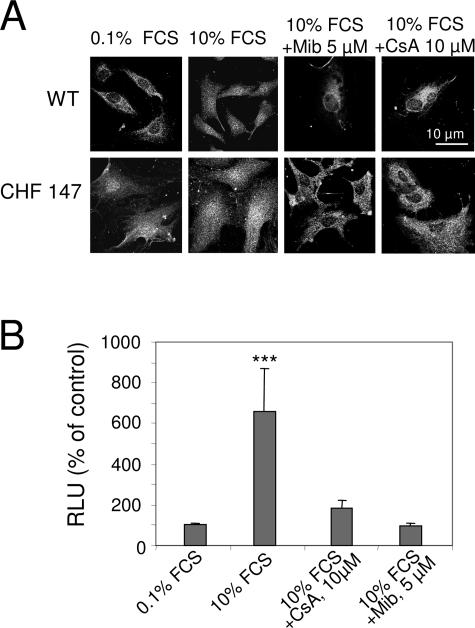
The fact that cyclosporine A blocks proliferation suggests the participation of the calcineurin/NFAT pathway. In freshly isolated CHF 147 VSMCs, NFAT was already located in the nucleus (ie, activated), as in proliferating WT VSMCs. Serum stimulation had no effect on NFAT activity in CHF 147 VSMCs (Figure 6A). After treatment of WT VSMCs with mibefradil (5 μmol/L) or CsA (10 μmol/L) for 24 hours in the presence of 10% FCS, NFAT was arrested in the cytosol. Mibefradil and CsA also both inhibited the activity of an NFAT-driven luciferase construct transfected into WT VSMCs (Figure 6B). These data show that NFAT is spontaneously active in CHF 147 VSMCs and suggest the involvement of enhanced Ca2+ channel entry sensitive to mibefradil.
Figure 6.
Involvement of NFAT in VSMC proliferation. A: Confocal immunofluorescence with anti-NFAT showing its cytosolic or nuclear localization in VSMCs. B: Promoter-reporter assay of NFAT transcriptional activity in WT VSMCs. Cells were transfected with NFAT-Luc and cultured for 48 hours without FCS. In A and B, cells were then treated with 10% FCS alone or together with 5 μmol/L mibefradil (Mib) or 10 μmol/L CsA for 5 hours. RLU, relative luminescence units. The bars represent mean ± SEM of three experiments in triplicate. ***P < 0.001 versus control (0.1% FCS).
VSMCs from CHF 147 Undergo Apoptosis
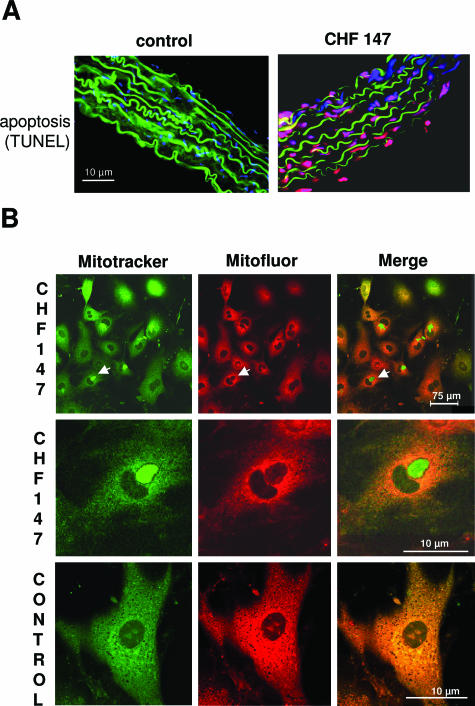
The atrophic zones observed in the media of CHF147 aorta prompted us to screen for apoptotic cells with the terminal deoxynucleotidyl transferase dUTP nick-end labeling method. No apoptosis was observed in WT aortas, whereas CHF 147 aortas contained abundant apoptotic cells distributed in patches along the aorta (Figure 7A). The percentages of apoptotic cells in freshly dissociated CHF 147 and WT VSMCs were 29.7 ± 5.6 and 4.3 ± 1.3%, respectively (P < 0.05). When cultured for 3 days with 10% serum, the proportion of apoptotic cells was 24.2 ± 5.2% in CHF 147 VSMCs and 1.7 ± 1.7% in WT VSMCs (P < 0.05).
Figure 7.
Apoptosis of CHF 147 VSMCs. A: Analysis of apoptosis in thoracic aorta cross-sections by the terminal deoxynucleotidyl transferase dUTP nick-end labeling method. The media are identified by elastin autofluorescence (green), apoptotic cells are terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive (red), and nuclei are marked by Hoechst staining (blue). B: Mitochondrial aggregation in cultured CHF 147 VSMCs. Confocal microscopy of live cells double-immunolabeled with MitoTracker Green and MitoFluor Red was used to assess the mitochondrial architecture and mitochondrial membrane potential changes. The green and red images were superimposed to visualize mitochondria that had lost their Δm.
Mitochondrial dysfunction is a major cause of apoptosis and is associated with cellular redistribution of mitochondria.32 We used MitoTracker Green to locate mitochondria and MitoFluor Red to measure their membrane potential. Aggregation of mitochondria near the nucleus was observed in 25% of CHF 147 VSMCs but in no WT cells (Figure 7B, left panel). These mitochondria were not labeled with MitoFluor Red, indicating that they were inactive (Figure 7B, middle and right). Merge images showed the colocalization of MitoTracker Green and MitoFluor Red signals in WT cells, whereas in CHF 147 VSMCs, the red and green signals were superimposed in some cells but clearly distinct in others.
Discussion
We demonstrate that the δ-sarcoglycan deficiency in myopathic CHF 147 hamsters leads to peripheral vascular smooth muscle disorders characterized by anarchic proliferation and apoptosis. This vascular remodeling is associated with an increased transmembrane Ca2+ entry and with activation of a Ca2+-dependent transcription pathway.
Vasospasm of small arteries such as coronary arteries is well documented in δ-sarcoglycan mutant animals,5,33,34,35 but VSMC dysfunction is not the primary cause of δ-sarcoglycan-deficient muscular dystrophy.2,36,37,38 Ca2+ channel blockers such as verapamil and mibefradil suppress vasospasm and improve cardiac function.2,35,39,40,41 Here, we describe another vascular disorder in this genetic disease, characterized by media remodeling of large vessels.
Several arguments indicate that, in CHF 147 hamsters, VSMC proliferation is due to increased calcium entry. First, the Ca2+-dependent calcineurin/NFAT-signaling pathway was activated as in control proliferating cells. Second, voltage-independent Ca2+ channel activity was markedly enhanced in VSMCs of δ-sarcoglycan-deficient animals and also in proliferating WT cells. These Ca2+ channels resemble basal Ca2+ channels described in cardiac myocytes (B-type channels), which are characterized by high conductance (>20 pS) and by their permeability to Ca2+ and Ba2+. They are inhibited by La3+, AlF3, eosin, and, as shown here, by mibefradil, a drug that blocks several calcium channels, including Cav1.2 L-type Ca2+ channels42,43 and store-operated channels.44 Third, VSMC proliferation and NFAT translocation to the nucleus were both suppressed by mibefradil, suggesting a link between enhanced Ca2+ channel activity and phenotypic changes.
Mibefradil prevented VSMC proliferation in vitro and neointima formation in a carotid balloon injury model in rats.45 It is unlikely that the antiproliferative effect of mibefradil is due to TTCC. TTCC were shown to control proliferation of human pulmonary myocytes,46 but the absolute magnitude of whole-cell inward currents was small (5 ± 2 pA) compared with the currents measured in the present study. In addition, in our study Ca2+ entry was voltage-independent, whereas in Rodman’s study the current was activated at −40 mV. Moreover, mibefradil inhibited TTCC expression and proliferation,47 whereas functional voltage-gated TTCC could not be detected in these tumor cell lines.48 Thus, inhibition of proliferation by mibefradil was due to an effect independent of voltage-gated channels.48 Furthermore, overexpression of TTCC generated by the human α1G and α1H subunits in human embryonic kidney 293 cells increased Ca2+ influx but not proliferation.49
Evidence of abnormal voltage-independent basal Ca2+ current in dystrophin-lacking skeletal muscle fibers has already been reported.12 In this latter case, the current was attributed to SOC activation. Moreover, minidystrophin gene transfer normalizes SOC activity.12 An increase in SOC activity has also been found during VSMC proliferation,50,51 and inhibition by RNA silencing or by pharmacological agents (carboxyamidotriazole and 2-aminoethoxydiphenyl borate) strongly inhibits cell proliferation.52 In our study, carboxyamidotriazole and 2-aminoethoxydiphenyl borate inhibited VSMC proliferation, but only at high concentrations (50 μmol/L) at which they are less specific for these channels. Moreover, SOC, which have very low conductance (24 fS to 2 pS) and which are regulated by submembrane calcium, cannot account for the strong channel activity recorded here in highly calcium-buffered proliferating hamster VSMCs. However, we cannot exclude the participation of other Ca2+ entry processes. Given that mibefradil can nonspecifically block multiple channels, the strong inhibition of mibefradil on proliferation of VSMCs could be due to additive effects on multiple channels. This is supported by the observation that, in our study, mibefradil only blocks half of the activity of B-type channels at a higher dose than used to almost fully block VSMC proliferation.
Resting VSMCs from rat cerebral arteries show nearly continual Ca2+ influx, called “Ca2+ sparklets” due to the opening of single or clustered L-type Ca2+ channels in a voltage-independent manner at resting potential.53,54 It is unlikely that LTCC contribute significantly to the strong channel activity in CHF 147 VSMCs. Moreover, diltiazem or nifedipine had little effect on VSMC proliferation, in keeping with the absence of LTCC in proliferating cells.55,56,57 However, similarly to the data of Santana group,53,54 our results point to the role of calcium channel bursts in the regulation of calcium-dependent processes in VSMCs.
One major finding of this study is that increased mibefradil-sensitive Ca2+ entry is coupled to the transcription factor NFAT pathway in freshly isolated CHF 147 and WT VSMCs. Increased calcineurin/PP2B and NFAT activity has also been detected in ventricular myocytes from δ-sarcoglycan-deficient J2N-k and UM-X7.1 cardiomyopathic hamsters.58,59 NFAT is strongly required for VSMC proliferation. Indeed, NFATc3 is activated in rat VSMCs in vitro by agonists that lead to proliferation28,60,61,62,63 and after balloon-induced restenosis in vivo.24,25 Forced expression of the NFAT-competing peptide VIVIT blocked VSMC proliferation both in vitro and in vivo.24,62 Finally, NFAT is involved in the control of cyclin D1 and pRb, and its inhibition leads to cell cycle arrest in the G1 phase24
We also observed abundant apoptosis in the vascular wall of CHF 147 hamsters, accompanied by mitochondrial abnormalities. Apoptosis and mitochondrial dysfunction have been observed in several models of myopathies, such as γ-sarcoglycan-deficient mice,64 desmin-related cardiomyopathy,65 and collagen deficiency-related myopathy.66 An important feature of the mitochondrial dysfunction observed here in CHF 147 VSMCs is their redistribution and aggregation around the nucleus, possibly representing an early step of apoptosis.32,67 Mitochondria are dynamic organelles and their movements result in spatial rearrangement of ATP production and Ca2+ buffering. For instance, elevation of the global [Ca2+]c to 1 μmol/L results in almost complete loss of mitochondrial movement.68 An increase in mitochondrial Ca2+ is observed in dystrophin-lacking myocytes,12,69 whereas lowering calcium entry by minidystrophin expression results in shorter calcium transients and reduced calcium uptake by mitochondria.12 We have no evidence of a link between enhanced voltage-independent channel activity, mitochondria calcium loading and apoptosis. However, we have previously shown that B-type Ca2+ channels can modulate mitochondrial calcium loading during cardiac myocyte apoptosis induced by ceramide.70 The fact that both proliferation and apoptosis are observed in the same vessel suggests that the aortic vessel wall is able to regenerate at the difference of the myocardium.
In conclusion, we demonstrate that the mutation of a cytoskeleton protein affects the activity of voltage-independent Ca2+ channels and triggers the activation of Ca2+ signaling pathways in vascular smooth muscle cells, resulting in major changes in phenotype and survival. Such a mechanism may not be restricted to sarcoglycanopathy but may concern other diseases characterized by dystrophin disruption and sarcolemma instability such as heart failure.71
Footnotes
Address reprint requests to Anne-Marie Lompré, INSERM UMR S621, 91 bd de l’Hôpital, 75634 Paris Cedex 13, France. E-mail: lompre@chups.jussieu.fr.
Supported by Association Française contre les Myopathies (AFM) no. 10973 to A.M.L. L.L. and C.P. are postdoctoral fellows supported by AFM and the Fondation pour la Recherche Médicale and by AFM and Fondation Lefoulon-Delalande, respectively.
References
- Tsubata S, Bowles KR, Vatta M, Zintz C, Titus J, Muhonen L, Bowles NE, Towbin JA. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest. 2000;106:655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MT, Korcarz CE, Collins KA, Lapidos KA, Hack AA, Lyons MR, Zarnegar S, Earley JU, Lang RM, McNally EM. Secondary coronary artery vasospasm promotes cardiomyopathy progression. Am J Pathol. 2004;164:1063–1071. doi: 10.1016/S0002-9440(10)63193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, Masaki T, Toyo-oka T, Hanaoka F. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA. 1997;94:13873–13878. doi: 10.1073/pnas.94.25.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack AA, Cordier L, Shoturma DI, Lam MY, Sweeney HL, McNally EM. Muscle degeneration without mechanical injury in sarcoglycan deficiency. Proc Natl Acad Sci USA. 1999;96:10723–10728. doi: 10.1073/pnas.96.19.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MT, McNally EM. Sarcoglycans in vascular smooth and striated muscle. Trends Cardiovasc Med. 2003;13:238–243. doi: 10.1016/s1050-1738(03)00101-4. [DOI] [PubMed] [Google Scholar]
- Turner PR, Fong PY, Denetclaw WF, Steinhardt RA. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991;115:1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong PY, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990;250:673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol. 1999;515:859–868. doi: 10.1111/j.1469-7793.1999.859ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, Gailly P, Cognard C, Constantin B. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20:136–138. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Scheuer T, Catterall WA. Convergent regulation of skeletal muscle Ca2+ channels by dystrophin, the actin cytoskeleton, and cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2005;102:4191–4196. doi: 10.1073/pnas.0409695102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TY, Iwata Y, Sampaolesi M, Hanada H, Saito N, Artman M, Coetzee WA, Shigekawa M. Stretch-activated cation channels in skeletal muscle myotubes from sarcoglycan-deficient hamsters. Am J Physiol. 2001;281:C690–C699. doi: 10.1152/ajpcell.2001.281.2.C690. [DOI] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21:608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Marchand E, Constantin B, Balghi H, Claudepierre MC, Cantereau A, Magaud C, Mouzou A, Raymond G, Braun S, Cognard C. Improvement of calcium handling and changes in calcium-release properties after mini- or full-length dystrophin forced expression in cultured skeletal myotubes. Exp Cell Res. 2004;297:363–379. doi: 10.1016/j.yexcr.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Friedrich O, Both M, Gillis JM, Chamberlain JS, Fink RH. Mini-dystrophin restores L-type calcium currents in skeletal muscle of transgenic mdx mice. J Physiol. 2004;555:251–265. doi: 10.1113/jphysiol.2003.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignard JF, Harricane MC, Menard C, Lory P, Nargeot J, Capron L, Mornet D, Richard S. Transient down-regulation of L-type Ca(2+) channel and dystrophin expression after balloon injury in rat aortic cells. Cardiovasc Res. 2001;49:177–188. doi: 10.1016/s0008-6363(00)00210-8. [DOI] [PubMed] [Google Scholar]
- Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, Lompre AM. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Lompré AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- Eggermont JA, Wuytack F, Verbist J, Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990;271:649–653. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty I, Robert M, Villaz M, De Jongh K, Lai Y, Catterall WA, Ronjat M. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc Natl Acad Sci USA. 1994;91:2270–2274. doi: 10.1073/pnas.91.6.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL, Capiod T, Lompre AM. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92:1115–1122. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- Coulombe A, Lefevre IA, Baro I, Coraboeuf E. Barium- and calcium-permeable channels open at negative membrane potentials in rat ventricular myocytes. J Membr Biol. 1989;111:57–67. doi: 10.1007/BF01869209. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Coraboeuf E, Ghazi A, Coulombe A. Divalent cation channels activated by phenothiazines in membrane of rat ventricular myocytes. J Membr Biol. 1995;147:147–158. doi: 10.1007/BF00233543. [DOI] [PubMed] [Google Scholar]
- Antoine S, Pinet C, Coulombe A. Are B-type Ca2+ channels of cardiac myocytes akin to the passive ion channel in the plasma membrane Ca2+ pump? J Membr Biol. 2001;179:37–50. doi: 10.1007/s002320010035. [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Factor SM, Minase T, Cho S, Dominitz R, Sonnenblick EH. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982;66:342–354. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH, Fein F, Capasso JM, Factor SM. Microvascular spasm as a cause of cardiomyopathies and the calcium-blocking agent verapamil as potential primary therapy. Am J Cardiol. 1985;55:179B–184B. doi: 10.1016/0002-9149(85)90629-0. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest. 2001;107:R1–7. doi: 10.1172/JCI11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Sawatzki SM, Barresi R, Schmainda KM, Allamand V, Michele DE, Campbell KP. Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc Natl Acad Sci USA. 2003;100:8910–8915. doi: 10.1073/pnas.1537554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MT, Allikian MJ, Heydemann A, Hadhazy M, Zarnegar S, McNally EM. Smooth muscle cell-extrinsic vascular spasm arises from cardiomyocyte degeneration in sarcoglycan-deficient cardiomyopathy. J Clin Invest. 2004;113:668–675. doi: 10.1172/JCI20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T, Nakazawa M, Nakauchi S, Yamazaki K, Shimamoto R, Urabe M, Nakata J, Hemmi C, Masui F, Nakajima T, Suzuki J, Monahan J, Sato H, Masaki T, Ozawa K, Toyo-Oka T. Rescue of hereditary form of dilated cardiomyopathy by rAAV-mediated somatic gene therapy: amelioration of morphological findings, sarcolemmal permeability, cardiac performances, and the prognosis of TO-2 hamsters. Proc Natl Acad Sci USA. 2002;99:901–906. doi: 10.1073/pnas.022641799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto H, Okamoto H, Watanabe M, Onozuka H, Yoneya K, Nakagawa I, Chiba S, Watanabe S, Mikami T, Abe K, Kitabatake A. Beneficial effect of myocardial angiogenesis on cardiac remodeling process by amlodipine and MCI-154. Am J Physiol. 1999;276:H1117–H1123. doi: 10.1152/ajpheart.1999.276.4.H1117. [DOI] [PubMed] [Google Scholar]
- Villame J, Massicotte J, Jasmin G, Dumont L. Effects of mibefradil, a T- and L-type calcium channel blocker, on cardiac remodeling in the UM-X7.1 cardiomyopathic hamster. Cardiovasc Drugs Ther. 2001;15:41–48. doi: 10.1023/a:1011158717901. [DOI] [PubMed] [Google Scholar]
- Paquette F, Jasmin G, Dumont L. Cardioprotective efficacy of verapamil and mibefradil in young UM-X7.1 cardiomyopathic hamsters. Cardiovasc Drugs Ther. 1999;13:525–530. doi: 10.1023/a:1007879704878. [DOI] [PubMed] [Google Scholar]
- Leuranguer V, Mangoni ME, Nargeot J, Richard S. Inhibition of T-type and L-type calcium channels by mibefradil: physiologic and pharmacologic bases of cardiovascular effects. J Cardiovasc Pharmacol. 2001;37:649–661. doi: 10.1097/00005344-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Bruderl B, Welling A, Hofmann F. Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res. 2006;98:105–110. doi: 10.1161/01.RES.0000197851.11031.9c. [DOI] [PubMed] [Google Scholar]
- Gackière F, Bidaux G, Lory P, Prevarskaya N, Mariot P. A role for voltage gated T-type calcium channels in mediating “capacitative” calcium entry? Cell Calcium. 2006;39:357–366. doi: 10.1016/j.ceca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Clozel JP, Iberg N, Buhler FR. Mibefradil prevents neointima formation after vascular injury in rats. Possible role of the blockade of the T-type voltage-operated calcium channel. Arterioscler Thromb Vasc Biol. 1995;15:1161–1165. doi: 10.1161/01.atv.15.8.1161. [DOI] [PubMed] [Google Scholar]
- Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005;96:864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- Panner A, Cribbs LL, Zainelli GM, Origitano TC, Singh S, Wurster RD. Variation of T-type calcium channel protein expression affects cell division of cultured tumor cells. Cell Calcium. 2005;37:105–119. doi: 10.1016/j.ceca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lu F, Chen H, Zhou C, Wu S. Is there a role for T-type Ca2+ channel in glioma cell proliferation? Cell Calcium. 2005;38:593–595; author reply 597. doi: 10.1016/j.ceca.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, Lory P. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett. 2000;478:166–172. doi: 10.1016/s0014-5793(00)01832-9. [DOI] [PubMed] [Google Scholar]
- Golovina VA. Cell proliferation is associated with enhanced capacitative Ca(2+) entry in human arterial myocytes. Am J Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca(2+) entry in human pulmonary artery myocytes during proliferation. Am J Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Mignen O, Brink C, Enfissi A, Nadkarni A, Shuttleworth TJ, Giovannucci DR, Capiod T. Carboxyamidotriazole-induced inhibition of mitochondrial calcium import blocks capacitative calcium entry and cell proliferation in HEK-293 cells. J Cell Sci. 2005;118:5615–5623. doi: 10.1242/jcs.02663. [DOI] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, Haller H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. 1998;12:593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- Kuga T, Kobayashi S, Hirakawa Y, Kanaide H, Takeshita A. Cell cycle-dependent expression of L- and T-type Ca2+ currents in rat aortic smooth muscle cells in primary culture. Circ Res. 1996;79:14–19. doi: 10.1161/01.res.79.1.14. [DOI] [PubMed] [Google Scholar]
- Richard S, Neveu D, Carnac G, Bodin P, Travo P, Nargeot J. Differential expression of voltage-gated Ca(2+)-currents in cultivated aortic myocytes. Biochim Biophys Acta. 1992;1160:95–104. doi: 10.1016/0167-4838(92)90042-c. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S, Saito N, Watano K, Igarashi K, Tagami S, Shima H, Kikuchi K. Defect of delta-sarcoglycan gene is responsible for development of dilated cardiomyopathy of a novel hamster strain, J2N-k: calcineurin/PP2B activity in the heart of J2N-k hamster. J Biochem (Tokyo) 2003;134:269–276. doi: 10.1093/jb/mvg140. [DOI] [PubMed] [Google Scholar]
- Ambra R, Di Nardo P, Fantini C, Minieri M, Canali R, Natella F, Virgili F. Selective changes in DNA binding activity of transcription factors in UM-X7.1 cardiomyopathic hamsters. Life Sci. 2002;71:2369–2381. doi: 10.1016/s0024-3205(02)02020-9. [DOI] [PubMed] [Google Scholar]
- Stevenson AS, Gomez MF, Hill-Eubanks DC, Nelson MT. NFAT4 movement in native smooth muscle. A role for differential Ca2+ signaling. J Biol Chem. 2001;276:15018–15024. doi: 10.1074/jbc.M011684200. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Nishimatsu H, Satonaka H, Walsh K, Goto A, Omata M, Fujita T, Nagai R, Hirata Y. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T cell-dependent transcriptional activation in vascular myocytes. Circ Res. 2002;90:1004–1011. doi: 10.1161/01.res.0000017629.70769.cc. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A, Rao GN. A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation. Biochem J. 2002;368:183–190. doi: 10.1042/BJ20020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem. 2002;277:37756–37764. doi: 10.1074/jbc.M203596200. [DOI] [PubMed] [Google Scholar]
- Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. γ-Sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation. 2005;112:3451–3461. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- Haga N, Fujita N, Tsuruo T. Mitochondrial aggregation precedes cytochrome c release from mitochondria during apoptosis. Oncogene. 2003;22:5579–5585. doi: 10.1038/sj.onc.1206576. [DOI] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- Hénaff M, Antoine S, Mercadier JJ, Coulombe A, Hatem SN. The voltage-independent B-type Ca2+ channel modulates apoptosis of cardiac myocytes. FASEB J. 2002;16:99–101. doi: 10.1096/fj.01-038fje. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T, Kawada T, Nakata J, Xie H, Urabe M, Masui F, Ebisawa T, Tezuka A, Iwasawa K, Nakajima T, Uehara Y, Kumagai H, Kostin S, Schaper J, Nakazawa M, Ozawa K. Translocation and cleavage of myocardial dystrophin as a common pathway to advanced heart failure: a scheme for the progression of cardiac dysfunction. Proc Natl Acad Sci USA. 2004;101:7381–7385. doi: 10.1073/pnas.0401944101. [DOI] [PMC free article] [PubMed] [Google Scholar]