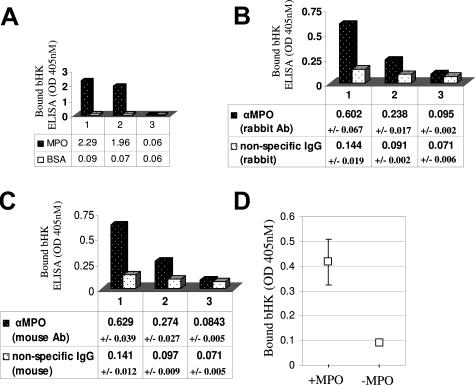
Figure 5.
MPO binds kininogen. A: Direct ELISA: column 1: biotinylated kininogen (bHK) binds immobilized MPO but not bovine serum albumin as detected by alkaline phosphatase-conjugated streptavidin; column 2: bHK binding is reduced in the presence of excess unlabeled kininogen (uHK); column 3: unlabeled kininogen alone was negative. B: Sandwich ELISA: further validation of the direct interaction between MPO and kininogen. Column 1: monoclonal MPO antibody-captured MPO binds bHK as detected by alkaline phosphatase-conjugated streptavidin; column 2: competition of bHK binding in the presence of excess uHK; column 3: unlabeled kininogen alone was negative. Shown are the averaged results of three independent trials ± SDs. C: Sandwich ELISA: validation using a second MPO antibody. Column 1: polyclonal MPO antibody-captured MPO binds bHK as detected by alkaline phosphatase-conjugated streptavidin; column 2: competition of bHK binding in the presence of excess uHK; column 3: unlabeled kininogen alone was negative. Shown are the results of three independent trials ± SDs. D: MPO captures bHK from plasma. HK-depleted plasma was replenished with bHK to physiological levels. Immobilized MPO was used as bait to capture bHK from plasma. Shown are the results (with SE bars) of three independent experiments.