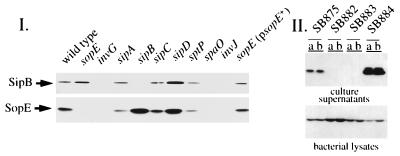
Figure 2.
Western blot analysis of SopE secretion. (I) Effect of targets and components of the centisome 63 type III secretion system on SopE secretion. Proteins from culture supernatants of the wild-type S. typhimurium SL1344 and the isogenic strains with nonpolar mutations in sopE (SB757), invG (SB161), sipA (SB225), sipB (SB169), sipC (SB220), sipD (SB221), sptP (SB237), spaO (SB302), or invJ (SB303), as well as from SB757 complemented with pSB1130 (which carries sopE) were resolved on an SDS/12% polyacrylamide gel, transferred to a nitrocellulose membrane, and sequentially probed with antibodies directed against SopE and SipB. Reprobing for the intracellular marker protein 6-phosphogluconate dehydrogenase verified that the observed pattern of supernatant proteins was not due to bacterial lysis (data not shown). (II) Secretion of epitope-tagged SopE. Culture supernatant proteins and whole-cell lysates (duplicate independent samples, a and b) from a wild-type S. typhimurium strain expressing, under the control of its native promoter, a chromosomally encoded, M45-epitope-tagged SopE (SB875), and its isogenic derivatives carrying mutations in invG (SB882), invC (SB883), or sipD (SB884), were separated on an SDS/12% polyacrylamide gel, transferred to nitrocellulose, and probed with a mouse monoclonal antibody directed to the epitope tag.