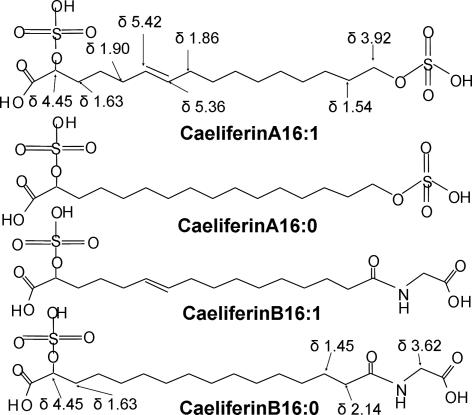
Fig. 3.
The structures of caeliferin A16:1, and A16:0 and caeliferin B16:1 and B16:0. The H-NMR chemical shifts were obtained for isolated natural compounds dissolved in D2O. Caeliferin A16:1 = (E)-2,16 disulfooxy-6-hexadecenoic acid; caeliferin A16:0 = 2,16 disulfooxyhexadecanoic acid; caeliferin B16:1 = N-[(E)-15-sulfooxy,15-carboxy-10-pentadecenoyl] glycine; and caeliferin B16:0 = N-(15-sulfooxy,15-carboxy pentadecanoyl) glycine.