Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is the aetiological agent of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The objective of this study is to identify which ex vivo and in vivo markers are associated independently with HAM/TSP in a Peruvian population. Eighty-one subjects (33 men/48 women) were enrolled: 35 presented with HAM/TSP, 33 were asymptomatic HTLV-1 carriers (ACs) and 13 were HTLV-1-seronegative controls (SCs). Ex vivo markers included T cell proliferation and Th1 [interferon (IFN)-γ], Th2 [interleukin (IL)-4, IL-5], proinflammatory [tumour necrosis factor (TNF)-α] and anti-inflammatory (IL-10) cytokine production in non-stimulated peripheral blood mononuclear cell (PBMC) cultures. In vivo CD4+ T cell count, markers of Th1 [interferon-inducible protein (IP)-10] and Th2 (sCD30) activity in plasma and HTLV-1 proviral load in PBMCs were also evaluated. In univariate analysis, several markers, including T cell proliferation, IFN-γ, IP-10, sCD30 and proviral load were associated with HAM/TSP, but in a multiple logistic regression analysis only the proviral load remained associated significantly with disease manifestation [adjusted OR 9·10 (1·24–66·91)]. Our findings suggest that HAM/TSP is associated primarily with proviral load, whereas the observed association with some immune markers seems secondary.
Keywords: cytokines, human T-lymphotropic virus 1, tropical spastic paraparesis, Peru, proviral load
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) was the first human retrovirus described [1]. It has been estimated that HTLV-1 infects 10–20 million people worldwide. Clinical studies have revealed a high prevalence of HTLV-1 infection in south-western Japan, whereas moderate rates have been reported in the Caribbean, West Africa, Australia and Central/South America [2]. In South America, Brazil, Peru and Colombia show the highest rates of infection [3]. A study carried out in Peru by Sanchez Palacios et al. reported between 1 and 4% prevalence of HTLV-1 infection in women from three different ethnic groups: Indigenous, African American and Mestizo [4].
HTLV-1 is recognized as the aetiological agent of two distinct types of disease: adult T cell leukaemia/lymphoma (ATLL) and a chronic inflammatory disease known as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [5–8]. In a prospective cohort study, it was found that up to 4% of HTLV-1 infected people develop HAM/TSP [9]. Although the factors that trigger disease expression have not been elucidated fully, interactions between host genetic and immune factors, as well as viral factors, are clearly important in the pathogenesis [10–12].
HTLV-1 proviral load has been proposed as a marker to predict the clinical outcome of HTLV-1 infection. In most cases it remains stable for years after reaching an equilibrium ‘set point’, which does not fluctuate by more than two- to fourfold [13]. Nagai et al. found a median proviral load percentage of 5% in PBMCs from patients with HAM/TSP and of 0·3% in asymptomatic HTLV-1 carriers from a Japanese population [14].
Several reports have shown that HTLV-1 infection is characterized by spontaneous T cell proliferation and production of proinflammatory cytokines such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α when peripheral blood mononuclear cells (PBMCs) from infected subjects are cultured ex vivo in the absence of any stimulus [15,16]. In addition, studies performed by flow cytometry and intracellular cytokine staining showed a high production of IFN-γ, TNF-α and interleukin (IL)-2 in PBMCs from HAM/TSP patients compared to HTLV-1-seronegative controls. Cytokine production was attributed primarily to HTLV-1-specific CD8+ lymphocytes [17], as the percentage of proliferating CD8+ T cells is two to five times higher than of CD4+ T cells [18]. Furthermore, the up-regulation of IL-2R expression and of IL-2 production, observed in both CD4+ and CD8+ T-cells of HTLV-1-infected patients, could contribute to the spontaneous proliferation and cytokine production [19].
Clearly, most investigators concentrated on ex vivo cytokine production, whereas cytokine activity in vivo has not been studied in HTLV-1-infected patients. Nevertheless, Th1 and Th2 serological markers, including IFN-γ-inducible protein-10 (IP-10) and soluble CD30 (sCD30), have been assessed in various infections [20–25]. Interferon-inducible protein (IP)-10 is a member of the CXC chemokine superfamily, which attracts activated Th1 cells and natural killer (NK) cells through interaction with CXC chemokine receptor 3 (CXCR3) [26]. With regard to CD30, it has been classified as a member of the tumour necrosis factor and nerve growth factor receptor superfamily. CD4+ and CD8+ T cells that produce cytokines associated with a Th2 phenotype can express CD30 on their surface [27,28].
It is still unclear why some HTLV-1-infected individuals develop a specific associated disease while others remain asymptomatic. The aim of the present study is to evaluate the relationship between immune markers, proviral load and disease expression. Specific attention is paid to plasma levels of IP-10 and sCD30 as well as to spontaneous ex vivo production of Th1 versus Th2 cytokines as potentially specific markers of HAM/TSP.
Materials and methods
Subjects and cells
Blood samples were obtained from 68 consecutive HTLV-1-infected patients in a clinical cohort study at the Institute of Tropical Medicine ‘Alexander von Humboldt’ in Lima, Peru, as well as 13 HTLV-1-seronegative controls (SCs) (uninfected laboratory students and uninfected relatives of HTLV-1-infected patients). The study protocol was approved by the Universidad Peruana Cayetano Heredia Research Ethics Committee. Written informed consent was obtained from all participants. HTLV-1 infection was determined by enzyme-linked immunosorbent assay (ELISA) (Sanofi Pasteur/Bio-Rad Laboratories, CA, USA or Cambridge Biotech Corp., MA, USA) and confirmed by Western blot (Genelabs Diagnostics, Singapore) or line immunoassay (INNO-LIA™ HTLV I/II Score; Innogenetics, Ghent, Belgium). The diagnosis of HAM/TSP was made by an expert physician according to World Health Organization criteria [29]. PBMCs were isolated from ethylenediamine tetraacetic acid (EDTA)-anti-coagulated peripheral blood via density gradient centrifugation on Ficoll-Hypaque (Amersham, Uppsala, Sweden), and washed three times with Hanks's buffered salt solution (Gibco, Paisley, Scotland, UK). All cells were resuspended in RPMI-1640 medium (Gibco) supplemented with 5% pooled human serum (PHS) obtained from uninfected laboratory students, 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco), further referred to as complete medium.
Proliferation assays
To evaluate the spontaneous T cell proliferation, PBMCs were cultured in 96-well U-bottomed plates (Falcon, Becton Dickinson, San Diego, CA, USA) in complete medium at 2 × 105 cells per well. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 3 days, without any additional stimulation. Afterwards, 0·4 µCi [3H]-thymidine (Sigma-Aldrich, St Louis, MO, USA) was added to each well for the last 5 h of incubation. The cells were harvested on filter paper (Filtermat A, Perkin Elmer, Turku, Finland), washed extensively and liquid scintillation mixture (Sigma-Aldrich) was added. Incorporated [3H]-thymidine was measured with a 1205 Betaplate Liquid Scintillation Counter (Wallac, Turku, Finland).
Cytokine determination
To evaluate the spontaneous production of cytokines (IFN-γ, TNF-α, IL-4, IL-5 and IL-10), 4 × 105 PBMCs per well were cultured as described in the previous section. Supernatants were collected on day 3 and stored at − 20°C. Cytokines levels were measured by sandwich ELISA technique using commercial kits (PharMingen, Erembodegem, Belgium). Plasma levels of IP-10 were evaluated using ELISA (R&D System, MN, USA) and sCD30 was measured in plasma samples using sCD30 module set (Bender MedSystems, Vienna, Austria).
Flow cytometry analysis
For enumeration of the CD4+ T cell subpopulation, whole blood was immunostained with saturating concentrations of combinations of anti-CD45-peridinin chlorophyll protein (PerCP), anti-CD3-fluorescein isothiocyanate (FITC) and anti-CD4-phycoerythrin (PE) (Becton Dickinson, Erembodegem, Belgium), according to the manufacturer's instructions. The red cells were removed with lysing solution (Becton Dickinson, San Diego) and the white cells were fixed with a 0·5% paraformaldehyde solution. Flow cytometric analysis was performed using a fluorescence activated cell sorter (FACS)Calibur (Becton Dickinson, San Diego). Data from 10 000 white blood cells were acquired and analysed using CellQuest software (Becton Dickinson, San Diego).
HTLV-1 proviral DNA quantification
HTLV-1 proviral load was determined by SYBR green-based real-time quantitative polymerase chain reaction (PCR) (qPCR) using the iCycler iQ instrument (Bio-Rad, Hercules, CA, USA). Genomic DNA was extracted from 5 × 106 PBMCs by a spin column procedure using the QIAamp blood DNA mini kit (Qiagen, Hilden, Germany) and used as the template for amplification.
The primers used for qPCR reactions (pX3-f: ACAAAGTTAACCATGCTTATTATCAGC and pX4-r: ACACGTAGACTGGGTATCCGAA) allowed amplification of an 80-base pairs (bp) fragment within the pX (tax/rex) region of the HTLV-1 proviral genome [30]. Human endogenous retrovirus 3 (ERV-3) DNA quantification was performed in parallel on all samples in order to determine the actual amount of DNA added and used as an endogenous reference to correct for variations due to differences in either PBMC count or DNA extraction. The ERV-3 target sequence was a 135-bp fragment in the envelope gene of the provirus, and the primers used for amplification were PHP10-F: CATGGGAAGCAAGGGAACTAATG, and PHP10-R: CCCAGCGAGCAATACAGAATTT [31]. qPCR reactions were performed in triplicate in a 25-µl reaction mixture consisting of 12·5 µl of the 2× iQ SYBR green Supermix reagent (Bio-Rad) (final 1× concentrations: 50 mM KCl, 20 mM Tris-HCl pH 8·4, 0·2 mM each deoxyribonucleoside triphosphate (dNTP), 3 mM MgCl2, 0·3 U of iTaq hot-start DNA polymerase, 10 nM fluorescein and SYBR green containing buffer), 0·2 µM of each primer and 5 µl of DNA sample (75–150 ng for clinical samples). For both HTLV-1 and ERV-3 DNA amplification reactions, an initial denaturation step at 95°C for 3 min was followed by 45 cycles at 95°C for 30 s and 60°C for 1 min. Data collection and analysis were performed with the iCycler iQ Optical System Software, version 3·0a (Bio-Rad).
Standard curves for proviral DNA quantification were generated using 10-fold serial dilutions (105−101 copies/reaction) of plasmid p4.39 (kindly provided by Thérèse Astier-Gin, Université Victor Ségalen, Bordeaux 2, France). This plasmid contains one copy of the HTLV-1 proviral genome, which was cloned from the HTLV-1 cell line 2060 [32]. During the experimental set-up, the plasmid standard DNA was diluted in the presence or absence of background gDNA from an HTLV-1-negative donor (roughly equivalent to 1·5 × 104 PBMC, in order to mimic the context of the clinical samples), and the sensitivity was compared by qPCR. It is important to mention that the presence or absence of background gDNA did not affect the sensitivity of HTLV-1 quantification because similar Ct values were obtained within a coefficient of variation (CV) of 2% for all tested dilutions of the standard plasmid DNA (data not shown). This finding corroborates results of a previous study [33]. In addition, the use of gDNA in our study lowered the PCR efficiency (86·3% compared to 94·6% in the absence of background gDNA).
The amount of proviral load was calculated as follows: [(average copy number of pX)/(average copy number of ERV-3)] × 2 × 104, and expressed as the number of HTLV-1 copies per 104 PBMCs. For this calculation, one copy of proviral DNA per HTLV-1-infected cell was assumed. A complete validation of this technique is described in a manuscript entitled ‘SYBR Green-based quantification of HTLV-1 proviral load in Peruvian patients with neurological disease and asymptomatic carriers: influence of clinical status, sex and familial relatedness’ (submitted for publication by Adavi V, Best I, Verdonck K et al).
Statistical analysis
Data were stored in Microsoft Excel and analysed with spss for Windows version 13·0 (SPSS, Inc., Chicago, IL, USA). In a first step, we compared the characteristics of HAM/TSP patients with those of asymptomatic HTLV-1 carriers through univariate analyses. T cell proliferation, IFN-γ, TNF-α, IL-4, IL-5, IL-10, IP-10, sCD30 and HTLV-1 proviral load were treated as continuous variables and evaluated with a two-tailed Mann–Whitney U-test. In a second step, we included all variables with a P-value of less than 0·1 in univariate analyses in a multiple logistic regression model. The final model was adjusted for proviral load and potential confounding variables (age, sex and relatedness to a HAM/TSP patient). Adjusted odds ratios (OR) with 95% confidence intervals (CIs) are reported for all independent variables.
Results
Subjects' characteristics
Thirty-five HAM/TSP patients and 33 ACs were included. Among HAM/TSP patients, 10 were men and 25 women with an age range between 22 and 72 years; ACs included 16 men and 17 women between 20 and 67 years. SCs included seven men and six women between 11 and 38 years. The SCs were younger than the ACs (P < 0·001), who were younger than HAM/TSP patients (P < 0·005). Sex ratios were not significantly different among the groups (P > 0·1), although it is evident that women were over-represented in the symptomatic patients. Clearly, older age and female gender are known to be associated with symptomatic disease [34–36]. Regarding the ethnic background, significant differences were observed only between SCs and HAM/TSP patients (P < 0·05), the latter group including relatively more subjects of Andean origin than mestizos or African Americans.
Ex vivo immune markers
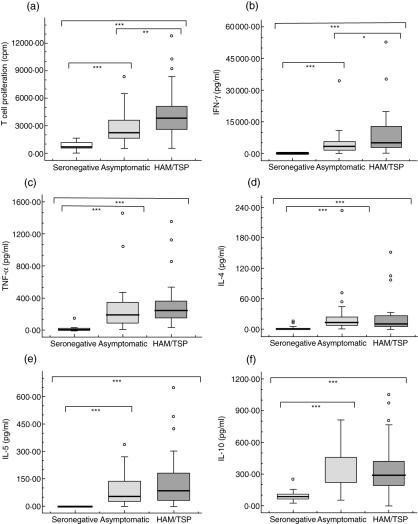
The spontaneous [3H]-thymidine incorporation ranged from 557 to 12800 counts per minute (cpm) in cultures of PBMCs from HAM/TSP patients, from 515 to 8831 cpm in those from ACs and from 102 to 1640 cpm in those from SCs (Fig. 1a). In univariate analysis, significant differences in the spontaneous T cell proliferation were observed between HAM/TSP patients and ACs (P < 0·005), HAM/TSP patients and SCs (P < 0·001), and ACs and SCs (P < 0·001) (Fig. 1a).
Fig. 1.
Ex vivo immune markers. Data represents spontaneous T cell proliferation expressed as counts per minute (cpm) and spontaneous cytokine production (pg/ml) in non-stimulated peripheral blood mononuclear cells (PBMCs), cultured for 72 h. Groups included human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients, asymptomatic HTLV-1 carriers and HTLV-1-seronegative controls. The Mann–Whitney U-test was used to evaluate differences among groups. Only significant differences are shown with: *P < 0·05, **P < 0·005, ***P < 0·001.
In order to assess if a specific cytokine production profile was associated with HAM/TSP, the spontaneous production of IFN-γ, TNF-α, IL-4, IL-5 and IL-10 was also measured in these cultures. Spontaneous production of all cytokines was much higher in HTLV-1 infected subjects (both ACs and HAM/TSP) compared to SCs, the latter showing negligible spontaneous cytokines production (Fig. 1b–f). Remarkably, only IFN-γ production was significantly higher in HAM/TSP compared to ACs (P < 0·05). Spontaneous IFN-γ production ranged from 217 to 53 129 pg/ml in HAM/TSP patients and from 27 to 34 511 pg/ml in ACs (Fig. 1b).
In vivo immune and viral markers
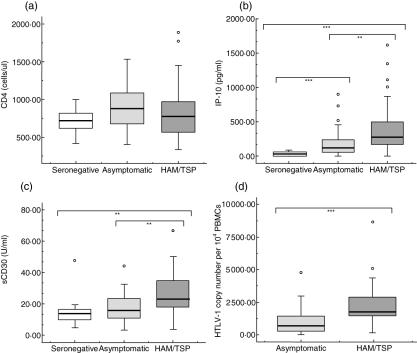
As shown in Fig. 2a, there was no difference in the CD4+ T cell count among the different groups. IP-10 and sCD30 were measured as serological markers of in vivo Th1 and Th2 activity. As shown in Fig. 2b, IP-10 levels ranged from 0 to 1618 pg/ml in HAM/TSP patients and 0–901 pg/ml in ACS, whereas sCD30 ranged from 3·52 to 66·64 U/ml in HAM/TSP patients and 3·60–44·20 U/ml in ACs (Fig. 2c). In univariate analysis we found a significant difference in both IP-10 and sCD30 between HAM/TSP patients and ACs (P < 0·005) (Table 1).
Fig. 2.
In vivo immune and virological markers. Data represented include absolute CD4 T cell counts (a) concentrations of interferon-inducible protein (IP)-10 (b) and sCD30 (c) in plasma and human T-lymphotropic virus type 1 (HTLV-1) proviral load expressed as HTLV-1 copy number per 104 peripheral blood mononuclear cells (PBMCs) (d). Groups included HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients, asymptomatic HTLV-1 carriers and HTLV-1-seronegative controls. The Mann–Whitney U-test was used to assess differences among groups. Levels of significance are: *P < 0·05, **P < 0·005, ***P < 0·001.
Table 1.
Immune and virological markers. Univariate and multivariate comparison between asymptomatic human T-lymphotropic virus type 1 (HTLV-1) carriers and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients.
| Asymptomatic HTLV-1 carriers | HAM/TSP patients | Univariate ORa (95% CI) | Multivariate ORa,b (95% CI) | |
|---|---|---|---|---|
| Sex ratio (male/female) | 16/17 | 10/25 | 2·35 (0·86–6·41) | 2·32 (0·62–8·62) |
| Relatedness to a HAM/TSP patient (yes/no) | 16/17 | 7/28 | 0·27 (0·09–0·78) | 0·19 (0·03–1·10) |
| Age (years) | 40 (20)c | 52 (21)c | 1·05 (1·01–1·10) | 1·04 (0·99–1·10) |
| CD4 (cells/µl) | 836 (436)c | 781 (410)c | 0·999 (0·998–1·001) | |
| T cell proliferation (cpm) | 2247 (1972)c | 3830 (2532)c | 1·000 (1·000–1·001) | 1·00 (1·00–1·00) |
| IFN-γ (pg/ml)d | 2958 (4110)c | 5312 (10 399)c | 2·75 (1·07–7·02) | 1·49 (0·34–6·47) |
| TNF-α (pg/ml)d | 193 (267)c | 246 (230)c | 2·87 (0·80–10·24) | |
| IL-4 (pg/ml)d | 13 (19)c | 10 (22)c | 0·76 (0·29–2·05) | |
| IL-5 (pg/ml)d | 53 (115)c | 85 (154)c | 1·42 (0·70–2·90) | |
| IL-10 (pg/ml) | 376 (243)c | 290 (234)c | 1·000 (0·998–1·002) | |
| IP-10 (pg/ml)d | 131 (197)c | 309 (345)c | 2·70 (1·04–7·02) | 1·17 (0·41–3·30) |
| sCD30 (U/ml) | 16 (13)c | 23 (19)c | 1·07 (1·02–1·13) | 1·02 (0·96–1·09) |
| Proviral load (HTLV-1 copy number/104 PBMC)d | 561 (1372)c | 1779 (1532)c | 8·87 (2·39–32·88) | 9·10 (1·24–66·91) |
OR: odds ratio, CI: confidence interval.
The ORs are expressed per unit increase in the independent variables.
Logistic regression model (enter approach) including: T cell proliferation, interferon (IFN)-γ, interferon-inducible protein (IP)-10, sCD30, proviral load, age, sex and relatedness to a HAM/TSP patient.
Median (interquartile range).
For univariate and/or multiple logistic regression analyses, the distributions of IFN-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4, IL-5, IP-10 and proviral load were normalized by log10 transformation. PBMC: peripheral blood mononuclear cells.
Finally, the proviral load ranged from 142 to 8641 HTLV-1 copies per 104 PBMCs in HAM/TSP patients compared to 1–4773 HTLV-1 copies per 104 PBMCs in ACs (Fig. 2d). In univariate analysis, this difference in proviral load between HAM/TSP patients and ACs was highly significant (P < 0·001) (Table 1).
Multiple logistic regression analysis
In summary, five of 10 markers studied (spontaneous T cell proliferation, spontaneous IFN-γ production, serum levels of IP-10 and sCD30 and proviral load in PBMCs) were significantly different between ACs and HAM/TSP. However, in the logistic regression analysis, taking into account also age, sex and relatedness to a HAM/TSP patient, the former four immune parameters showed no independent association with HAM/TSP [adjusted OR per unit increase in the independent variables: 1·00 (1·00–1·00), 1·49 (0·34–6·47), 1·17 (0·41–3·30) and 1·02 (0·96–1·09), respectively] (Table 1). Only the proviral load was associated independently with disease manifestation [adjusted OR 9·10 (1·24–66·91)].
Discussion
Various viral and immune markers are known to be associated with HTLV-1 seropositivity and/or the presence of the neurological disease HAM/TSP. These include in vivo proviral load and ex vivo non-stimulated T cell proliferation and cytokine production. From the existing literature, it is not clear which of these markers is associated primarily with HAM/TSP and which ones are secondary, reflecting the uncertainty over the question of whether the pathogenesis of HAM/TSP is driven primarily by high proviral load or by an inflammatory reaction against the virus. We critically re-evaluated these already established markers in a Peruvian cohort and in addition we included two serum factors IP-10 and sCD30, which could provide convenient parameters of in vivo Th1 and Th2 activity compared to the more cumbersome ex vivo culture measurements.
In univariate analysis, a significant difference between HAM/TSP patients and ACs was observed in ex vivo spontaneous T cell proliferation and IFN-γ production, as well as in vivo IP-10 and sCD30 plasma levels and proviral load in PBMCs (Table 1). The final regression model was adjusted by proviral load and potential confounding variables. After this analysis, no immune markers evaluated were found to be associated independently with HAM/TSP. Only proviral load remained associated significantly with disease (Table 1).
Previous studies have shown differences in the cellular immune response between HAM/TSP patients and asymptomatic HTLV-1 carriers; however, in most cases the immune parameters were not adjusted by proviral load. Two previous studies in Brazil reported spontaneous production of several cytokines such as IFN-γ, TNF-α, IL-5 and IL-10 by PBMCs from asymptomatic HTLV-1 carriers, which was not observed in cells from HTLV-1-seronegative controls. This observation already indicated that both Th1 and Th2 cytokines are elevated during HTLV-1 infection [15,16]. The latter study also compared HAM/TSP patients with asymptomatic HTLV-1 carriers and found a spontaneous high production of Th1 and proinflammatory cytokines such as IFN-γ and TNF-α in HAM/TSP patients compared to asymptomatic HTLV-1 carriers, but only IFN-γ production was significantly different between both groups [16]. These findings are in agreement with our results.
The relationship between HTLV-1 specific cellular immune response, presence of neurological disease and proviral load has been investigated in only a few studies. When comparing HAM/TSP patients to asymptomatic HTLV-1 carriers exhibiting a similar high proviral load, a study in Japan revealed a higher production of HTLV-1-specific IFN-γ and TNF-α in the Tax-expressing T cells of HAM/TSP patients [37]. Another study reported a significantly higher frequency of HTLV-1-specific IFN-γ-secreting CD4+ T cells in HAM/TSP patients compared to asymptomatic HTLV-1 carriers, but a significant correlation between proviral load and the frequency of HTLV-1-specific CD4+ T cells was not found [38]. These findings indicated that the association between HTLV-1 specific cellular immune response and proviral load remained to be clarified.
Other possible immune factors could be associated with disease manifestation. HTLV-1-specific CD8+ lymphocyte (CTL) responses contribute in part to control the proviral load and are associated with a lower risk of HAM/TSP [10–12,36]. A study showed that approximately 40–50% of the between-individual variation in proviral load was attributable to variation in the rate at which CTL cleared infected cells [39]. Host genetic factors implicated in the HTLV-1-specific CTL response have been associated with disease susceptibility among HTLV-1 carriers [40,41]. A case–control study carried out in southern Japan showed the protective effect of human leucocyte antigen (HLA) class I alleles against disease. The possession of HLA-A*02 or HLA-Cw*08 alleles was associated with a diminished risk of HAM/TSP patients and a lower proviral load in asymptomatic HTLV-1 carriers [40,41].
Markers of Th1 and Th2 activity in vivo such as IP-10 and sCD30, respectively, have not been studied in HAM/TSP patients so far. They have been investigated, however, in various other diseases [20–25]. An increase in IP-10 production has been reported during the course of several inflammatory diseases associated predominantly with a Th1 phenotype such as visceral leishmaniasis (VL) [20] and septic arthritis [21]. On the other hand, some studies have reported that activated Th2 cells and Th0 clones release sCD30 to the plasma during diseases associated with increased Th2 activity such as systemic sclerosis [22], Omenns' syndrome [25] and HIV patients who had experienced immune reconstitution after receiving highly active anti-retroviral therapy (HAART) [23]. Our present data indicate that both IP-10 and sCD30 are significantly more elevated in HAM/TSP patients compared to asymptomatic HTLV-1 carriers. Clearly, whereas the ex vivo data identified only the Th1 IFN-γ cytokine as discriminatory between asymptomatic HTLV-1 carriers and HAM/TSP, the in vivo measurement suggests that both Th1 and Th2 activity is relatively increased in neurological complication. Notwithstanding, all these immune markers appeared to be secondary to relative proviral load increase in HAM/TSP.
The use of proviral load as a marker to assess the risk of disease or prognosis in asymptomatic HTLV-1 carriers has been evaluated in some prospective studies [42,43]. In a cohort of 20 initially asymptomatic HTLV-1 carriers, it was shown that individuals who developed symptoms or inflammatory conditions such as HAM/TSP or uveitis presented a persistently higher proviral load than those individuals who remained asymptomatic [42]. Another prospective study of transfusion transmission in Jamaica followed 24 subjects who experienced HTLV-1 seroconversion. Early after transfusion, a 10-fold increase in proviral load was observed in an individual who developed HAM/TSP compared to those subjects who remained asymptomatic. However, a second individual presenting an earlier increase in proviral after transfusion did not develop HAM/TSP, suggesting that other factors might be influencing the expression of disease [43].
The pathogenesis of HAM/TSP is considered to be due to excess of T cell activation [11]. Logically, therefore, the risk of disease may be associated more closely with the amount of viral antigen rather than with the level of proviral DNA. A recent study showed indeed that high Tax expression was significantly associated with HAM/TSP, and that its expression level is a better predictor of disease status than proviral load. In addition, Tax expression showed a significant correlation with proviral load and was associated significantly with disease independently of proviral load. These findings suggest that Tax expression drives immune activation and HAM/TSP and that proviral load is a surrogate marker for Tax expression [44]. Clearly, whereas the quantitative evaluation of Tax expression is technically demanding, the measurement of proviral load, using real-time PCR, is relatively straightforward and therefore it could constitute a more clinically applicable parameter.
In conclusion, our investigation represents the first study that makes use of multiple logistic regression analysis to show that proviral load is associated independently with HAM/TSP, whereas ex vivo and in vivo immune markers, such as IFN-γ, IP-10 and sCD30, showed only an association with disease in univariate analysis.
Acknowledgments
We would like to thank the staff at the Institute of Tropical Medicine Alexander von Humboldt (IMTAvH), in particular Giovanni López, Afilio Tello and Juana Huertas for their valuable assistance in the attention of HTLV-1-infected patients, Dr Miguel Campos for help with the statistical analysis and Dr Thérèse Astier-Gin (CNRS UMR5097, Université Victor Ségalen, Bordeaux 2, France) who provided the HTLV-1 plasmid p4.39 for the proviral load assays. This study was supported by the Directorate-General for Development Cooperation (DGDC) of the Belgian Government (framework agreement 02, project 95501) and the Flemish Interuniversity Council (VLIR).
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–19. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de The BR. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9:381. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- 3.Gotuzzo E. Human T-cell lymphotropic virus-I in Latin America. Infect Dis Clin North Am. 2000;14:211–39. doi: 10.1016/s0891-5520(05)70225-7. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Palacios C, Gotuzzo E, Vandamme AM, Maldonado Y. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int J Infect Dis. 2003;7:132–7. doi: 10.1016/s1201-9712(03)90009-9. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematological features of 16 cases. Blood. 1977;50:481–92. [PubMed] [Google Scholar]
- 6.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 7.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–10. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 8.Gotuzzo E, Cabrera J, Deza L, et al. Clinical characteristics of patients in Peru with human T cell lymphotropic virus type 1-associated tropical spastic paraparesis. Clin Infect Dis. 2004;39:939–44. doi: 10.1086/423957. [DOI] [PubMed] [Google Scholar]
- 9.Orland JR, Engstrom J, Fridey J, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV outcomes study. Neurology. 2003;61:1588–94. doi: 10.1212/01.wnl.0000096011.92542.da. [DOI] [PubMed] [Google Scholar]
- 10.Bangham CR. The immune response to HTLV-1. Curr Opin Immunol. 2000;12:397–402. doi: 10.1016/s0952-7915(00)00107-2. [DOI] [PubMed] [Google Scholar]
- 11.Bangham CR. The immune control and cell-to-cell spread of human-T cell lymphotropic virus type I. J Gen Virol. 2003;84:3177–89. doi: 10.1099/vir.0.19334-0. [DOI] [PubMed] [Google Scholar]
- 12.Mosley AJ, Asquith B, Bangham C. Cell-mediated immune response to human T-lymphotropic virus type I. Viral Immunol. 2005;18:293–305. doi: 10.1089/vim.2005.18.293. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki T, Nakagawa M, Nagai M, et al. HTLV-1 proviral load correlates with progression of motor disability in HAM/TSP. analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol. 2001;7:228–34. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- 14.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-1 proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93. [Google Scholar]
- 15.Carvalho EM, Bacellar O, Porto AF, Braga S, Galvao-Castro B, Neva F. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic virus type-I-infected blood donors. J Acquir Immune Defic Syndr. 2001;27:1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiraku N, Ijichi S, Yashiki S, Osame M, Sonoda S. Cell surface phenotype of in vitro proliferating lymphocytes in HTLV-I-associated myelopathy (HAM/TSP) J Neuroimmunol. 1992;37:223–8. doi: 10.1016/0165-5728(92)90006-7. [DOI] [PubMed] [Google Scholar]
- 18.Sakai JA, Nagai M, Brennan MB, Mora C, Jacobson S. In vitro spontaneous lymphoproliferation in patients with human T-cell lymphotropic virus type I-associated neurological disease. predominant expansion of CD8+ T cells. Blood. 2001;98:1506–11. doi: 10.1182/blood.v98.5.1506. [DOI] [PubMed] [Google Scholar]
- 19.McGuire KL, Curtiss VE, Larson EL, Haseltine WA. Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J Virol. 1993;67:1590–9. doi: 10.1128/jvi.67.3.1590-1599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hailu A, Van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-γ, IFN-γ inducing cytokines, and IFN-γ inducible cytokines CXC chemokines in visceral Leishmaniasis. Am J Trop Med Hyg. 2004;71:561–7. [PubMed] [Google Scholar]
- 21.Proost P, Vynckier AK, Mahieu F, et al. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-γ and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol. 2003;33:3146–53. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- 22.Scala E, Pallotta S, Frezzolini A, et al. Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol. 2004;138:540–6. doi: 10.1111/j.1365-2249.2004.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane NM, Price P, Lee S, Stone SF, French MA. An evaluation of serum soluble CD30 levels and serum CD26 (DPPIV) enzyme activity as markers of type 2 and type 1 cytokines in HIV patientsreceiving highly active antiretroviral therapy. Clin Exp Immunol. 2001;126:111–6. doi: 10.1046/j.1365-2249.2001.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lienhardt C, Azzurri A, Amedei A, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002;32:1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Chilosi M, Facchetti F, Notarangelo LD, et al. CD30 cell expression and abnormal CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–34. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 26.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 27.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–6. [PubMed] [Google Scholar]
- 28.Manetti R, Annunziato F, Biagiotti R, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180:2407–11. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osame M. Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: Blattner WA, editor. Human retrovirology: HTLV. New York: Raven Press; 1990. pp. 191–7. [Google Scholar]
- 30.Nagai M, Brennan MB, Sakai JA, Mora CA, Jacobson S. CD8+ T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood. 2001;98:1858–61. doi: 10.1182/blood.v98.6.1858. [DOI] [PubMed] [Google Scholar]
- 31.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Meth. 2001;91:109–17. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 32.Nicot C, Astier-Gin T, Edouard E, et al. Establishment of HTLV-I-infected cell lines from French, Guianese and West Indian patients and isolation of a proviral clone producing viral particles. Virus Res. 1993;30:317–34. doi: 10.1016/0168-1702(93)90099-9. [DOI] [PubMed] [Google Scholar]
- 33.Dehée A, Césaire R, Désiré N, et al. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Meth. 2002;102:37–51. doi: 10.1016/s0166-0934(01)00445-1. [DOI] [PubMed] [Google Scholar]
- 34.Hanada S, Uematsu T, Iwahashi M, et al. The prevalence of human T-cell leukemia virus type I infection in patients with hematologic and nonhematologic diseases in an adult T-cell leukemia-endemic area of Japan. Cancer. 1989;64:1290–5. doi: 10.1002/1097-0142(19890915)64:6<1290::aid-cncr2820640620>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EL, Figueroa JP, Gibbs WN, et al. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am J Epidemiol. 1991;133:1114–24. doi: 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 36.Maloney EM, Cleghorn FR, Morgan OS, et al. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:167–70. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa Y, Saito M, Matsumoto W, et al. Different cytokine production in tax-expressing cells between patients with human T cell lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis and asymptomatic HTLV-1 carriers. J Infect Dis. 2003;187:1116–25. doi: 10.1086/368379. [DOI] [PubMed] [Google Scholar]
- 38.Goon PK, Igakura T, Hanon E, et al. Human T cell lymphotropic virus type I (HTLV-I)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I. J Immunol. 2004;172:1735–43. doi: 10.4049/jimmunol.172.3.1735. [DOI] [PubMed] [Google Scholar]
- 39.Asquith B, Mosley AJ, Barfield A, et al. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J Gen Virol. 2005;86:1515–23. doi: 10.1099/vir.0.80766-0. [DOI] [PubMed] [Google Scholar]
- 40.Jeffery KJ, Siddiqui AA, Bunce M, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–53. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffery KJ, Siddiqui AA, Bunce M, et al. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J Immunol. 2000;165:7278–84. doi: 10.4049/jimmunol.165.12.7278. [DOI] [PubMed] [Google Scholar]
- 42.Taylor GP, Tosswill JH, Matutes E, et al. Prospective study of HTLV-I infection in an initially asymptomatic cohort. J Acquir Immune Defic Syndr. 1999;22:92–100. doi: 10.1097/00042560-199909010-00012. [DOI] [PubMed] [Google Scholar]
- 43.Manns A, Miley WJ, Wilks RJ, et al. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J Infect Dis. 1999;180:1487–93. doi: 10.1086/315088. [DOI] [PubMed] [Google Scholar]
- 44.Asquith B, Mosley A, Heaps A, et al. Quantification of the virus–host interaction in human T lymphotropic virus I infection. Retrovirology. 2005;2:75. doi: 10.1186/1742-4690-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]