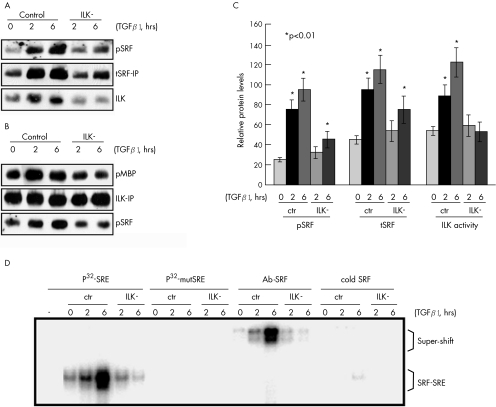
Figure 5 Transforming growth factor β1 (TGFβ1) induces serum response factor (SRF)‐binding activity to smooth muscle (SM) α‐actin serum response element (SRE) in an integrin‐linked kinase (ILK)‐dependent manner. (A) Immunoprecipitation with an anti‐SRF antibody (tSRF‐IP) showing that SRF is associated with ILK and inactivation of ILK (ILK−) prevents both TGFβ1‐induced SRF phosphorylation (pSRF) and total SRF protein upregulation (tSRF). (B) Immunoprecipitation with an anti‐ILK antibody (ILK‐IP) followed by a kinase assay using myelin basic protein (MBP) as a substrate confirming that SRF phosphorylation is associated with ILK activation. (C) Quantitative analysis based on 10 replicates. *p<0.01. Error bars represent SD. ctr, Control. (D) Electrophoretic mobility shift assay showing that TGFβ1 treatment increases SRF protein binding to smooth muscle α‐actin SRE in an ILK‐dependent manner. 10 μg protein lysate was incubated with 5 ng of either 32P‐labelled consensus smooth muscle α‐actin SRE oligonucleotide (P32‐SRE) or 32P‐labelled mutant SRE (P32‐mutSRE). For super‐shift assays, 2 μg of the anti‐SRF antibody (Ab‐SRF) was also included in the reaction. To confirm the specificity of the binding products, a 50‐fold ( = 250 ng) excess of unlabelled consensus SRE oligonucleotide (cold SRE) was added to the reaction to compete with the 32P‐labelled SRE for SRF binding.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.