Figure 2.
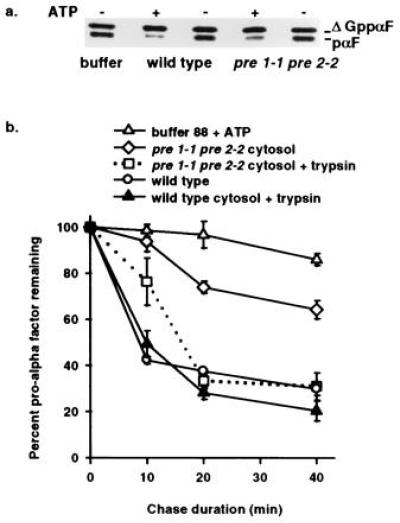
In vitro assay demonstrates ATP and proteasome-dependent ERAD and reveals export of pαF from ER-derived microsomes. (a) Phosphorimage of a 30-min posttranslocation chase incubation performed in the presence (+) or absence (−) of ATP with either proteasome mutant (pre 1-1 pre 2-2) or isogenic wild-type (wild type) cytosol. Radiolabeled ΔGppαF was translocated into wild-type microsomes and the signal sequence cleaved to generate unglycosylated pαF, a substrate for ERAD in vivo (20) and in vitro (8). (b) First-order decay curves generated with averaged values from at least three independent experiments indicate that pαF is stabilized in the presence of pre 1-1 pre 2-2 proteasome mutant cytosol. Posttranslocation chase incubations (0, 10, 20, and 40 min) in the presence of either wild-type cytosol (wild type), mutant cytosol (pre 1-1 pre 2-2 cytosol), or buffer (buffer 88 + ATP) were treated with or without trypsin before trichloroacetic acid precipitation, and samples were resolved on 18% SDS/urea-PAGE and analyzed as in Fig. 1. Degradation of pαF by trypsin treatment (0.25 g/ml) indicates that the protein substrate has been exported from microsomes, while protease protection indicates membrane-occluded pαF. All reactions were performed in the presence of ATP.
