Figure 2.
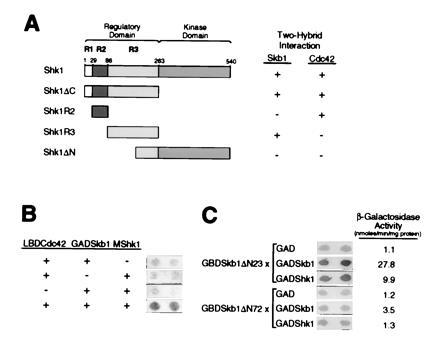
Analysis of two-hybrid interactions between Skb1, Shk1, and Cdc42. (A) Skb1 forms a complex with the R3 subdomain of Shk1. Pairs of two-hybrid fusion proteins were tested for the ability to form complexes as indicated. The domains of Shk1 tested were as follows: Shk1ΔC, residues 1 to 262; Shk1R2, residues 29 to 85; Shk1R3, residues 86 to 262; and Shk1ΔN, residues 189-540. Shk1, Shk1R3, and Shk1ΔN were expressed as LBD fusions; Shk1R2 was expressed as a GBD fusion; and Shk1ΔC was expressed as a GAD fusion. Skb1 and Cdc42 were expressed as GAD fusions for testing against LBD-Shk1, LBD-Shk1R3, and LBD-Shk1ΔN. For testing against GAD-Shk1ΔC, Cdc42 and Skb1 were expressed as LBD and GBD fusions, respectively. The X-Gal filter assay was used for measuring β-gal activity. The detection (+) or not (−) of β-gal is indicated. Two-hybrid interactions were detected between Skb1 and Shk1, Shk1R3, and Shk1ΔC, and between Cdc42 and Shk1, Shk1R2, and Shk1ΔC. (B) Evidence that Skb1, Cdc42, and Shk1 form a ternary complex in vivo. S. cerevisiae strain L40 was transformed (+) or not (−) with plasmids expressing Skb1 fused to GAD (GAD-Skb1), Cdc42 fused to LBD (LBD-Cdc42), and/or c-myc epitope-tagged Shk1 (MShk1) and assayed for β-gal expression by filter assay (shown at right). Complex formation between Skb1 and Cdc42 is bridged by MShk1, suggesting that the three proteins form a ternary complex in vivo. (C) Evidence that Skb1 homomerization is required for interaction with Shk1. Skb1ΔN23 (Skb1-1) and Skb1ΔN72 GBD fusion proteins were tested for their ability to form complexes with GAD, GAD-Skb1, or GAD-Shk1 as indicated. β-gal activity was measured using both X-Gal filter assays and liquid assays of cell extracts. Values at right of panel indicate β-gal activity (nmoles/min per mg protein) as measured by liquid assay.
