Figure 3.
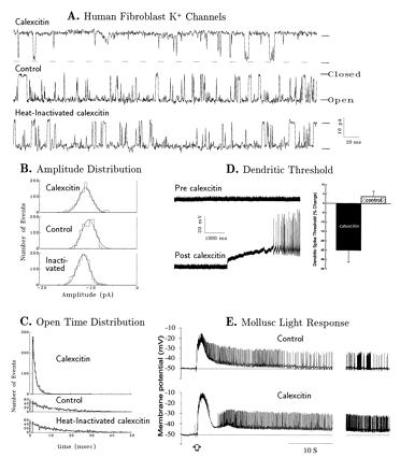
(A) Single-channel current traces of K+ channel activity in an excised inside-out membrane patch of human skin cultured fibroblast recorded in the absence of CE 7 min after excision of the membrane patch (middle trace), at 10 min after addition of heat-inactivated CE (bottom trace), and at 3 min after addition of intact CE (top trace). The channel shown was typical of those recorded at −40 mV. Pipette solution was 140 mM KCl/2 mM CaCl2/1 mM MgCl2/10 mM Hepes (NaOH)/0.001 mM tetrodotoxin, pH 7.4; bathing solution was 150 mM NaCl/5 mM KCl/2 mM CaCl2/1 mM MgCl2/10 mM Hepes (NaOH), pH 7.4. Addition of protein was made by a 20-μl plastic pipette near the tip of the patch micropipette as 10-μl aliquots of CE in 10 mM imidazole·HCl. Petri dish contained 2 ml of bath solution. CE markedly reduced the mean open time (τopen) and probability of openings. (B) Amplitude distributions of unitary K+ current records were fitted with a Gaussian function. Unitary current peak amplitudes were −10.6, −11.0, and −9.9 pA after addition of CE and heat-inactivated CE, and no addition, respectively. There were no significant differences in distribution among the three groups. (C) Open time distributions for the data collected during 30-s periods after excision of the membrane (as mentioned in A) were fitted to a single exponential. Exponential decay constants for open time distribution were 1.4, 12.0, and 12.5 ms after addition of CE and heat-inactivated CE, and no addition, respectively. CE application markedly reduced the open times of the K+ channel. (D) Membrane excitability increases due to CE. (Left) Passively propagated somatic spikes (small amplitude) occurred spontaneously in most cells, including the one illustrated here, before CE injection (pre). Spontaneous local dendritic calcium spikes (large amplitude) did not occur in some cells (upper trace, pre-calexcitin) but then did occur after injection of CE (post) through the recording electrode. In Purkinje cells hyperpolarized 20 mV below the membrane potential for somatic spikes, calcium spikes could be elicited by lower levels of injected positive current pulses after injection of CE. (Right) Mean percent change in the threshold current required to elicit local calcium spikes was lower for CE-injected cells vs. controls injected with heat-inactivated CE or 3 M potassium acetate (P < 0.001, Student’s t test). (E) Intracellular recordings of the Hermissenda type B photoreceptor response to a 1-s flash of light (103 erg/cm2·sec) before (Upper) and after (Lower) injection of purified cloned CE. The Hermissenda photoreceptors were isolated and submerged in artificial sea water (430 mM NaCl/10 mM KCl/10 mM CaCl2/50 mM MgCl2/10 mM Hepes Na, pH 7.4). The CE (intraelectrode concentration, 364 nM) was brought to 1 M in KAc (pH 7.4) and injected (3 min, 2 nA) into the photoreceptor with the recording electrode. Recordings were obtained using intracellular amplifier (Axopatch 2A), digitized at 50 Hz (Digidata 1200), and analyzed by computer. The normal light response returned to the original resting potential within a minute (Upper). Injection of CE (n = 5 cells) enhanced the light response and the cell remained depolarized for more than 5 min (Lower, only 1 min is represented). Injection of heat-inactivated CE (n = 6 cells) using the above protocol did not alter the light response. Time scale at right is compressed by 3×.
