Figure 2.
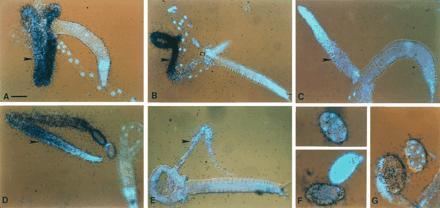
In situ hybridization to C. elegans adults and embryos. glh-1 hybridization to a splayed hermaphrodite (A) and male (B) using a 253-nt antisense probe from the glh-1-specific 5′ EcoRI–BamHI fragment (18). (C) Sense strand of the same glh-1 probe as a negative control. The gonads of the splayed worms are indicated with arrowheads. The 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei are blue. All glh-1 slides were hybridized with 5 × 105 dpm and exposed for 7 days. (D and E) Antisense glh-2 RNA hybridization to a hermaphrodite (D) and male (E). The probe used was 340 bp long, including 130 bp of the 3′-most coding region and the entire 210-bp glh-2 3′ UTR, minus the poly(A) tail. This probe was determined to be specific for glh-2 by both Northern and Southern blot analyses (data not shown). Exposures for glh-2 were 14 days, using 106 dpm. This glh-2 signal results from use of 2-fold higher probe concentration and exposure relative to the glh-1 conditions. (F) Antisense glh-1 hybridization to whole-mount embryos. From top to bottom, the embryo stages are: 8-cell stage, >60-cell stage, and 1-cell stage. (G) Antisense glh-2 hybridization to a whole mount 1-cell embryo (Left) and a 12- to 14-cell embryo (Right). Embryos were exposed for 7 days with 106 dpms. (Bars: A–E, 50 μm; F and G, 20 μm.)
