Figure 3.
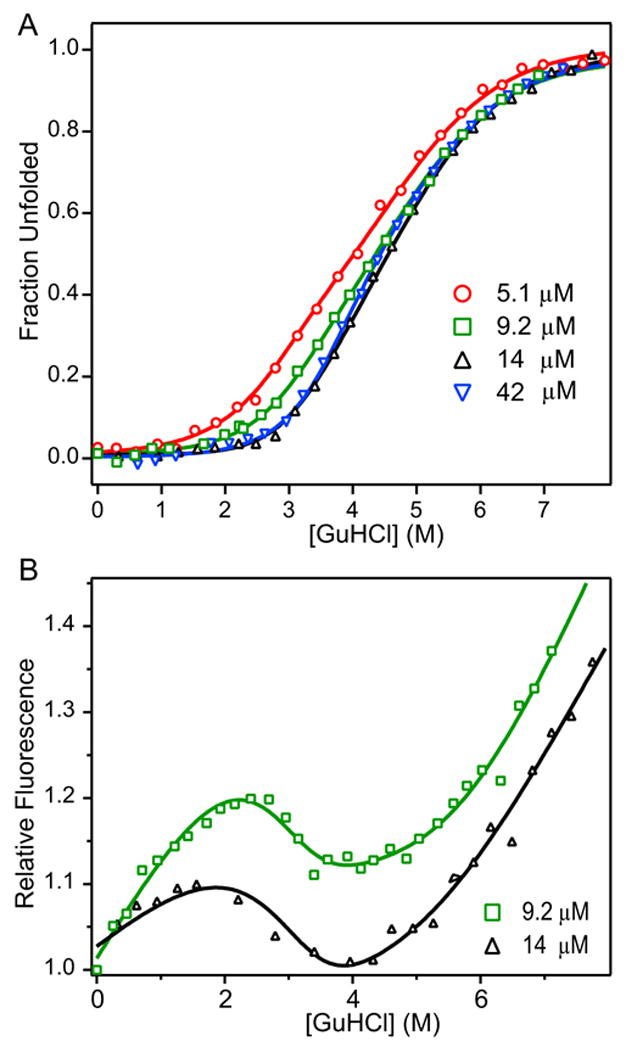
Normalized ellipticity (A) and relative fluorescence (B) of C321S A4 protein as a function of GuHCl globally fitted to three-state dimeric model with monomeric intermediate. Normalized molar residue ellipticity (A) and relative fluorescence (B) of C321S A4 at 5.1 μM (red circles), 9.2 μM (green squares), 14 μM (black triangles), and 42 μM (blue inverted triangles) vs. GuHCl concentration. Data were fit to a three-state dimeric model of protein unfolding with a partially structured monomeric intermediate (Scheme 3, equation 4).
