Figure 1. Alternative maturation pathways for the HSV-1 procapsid.
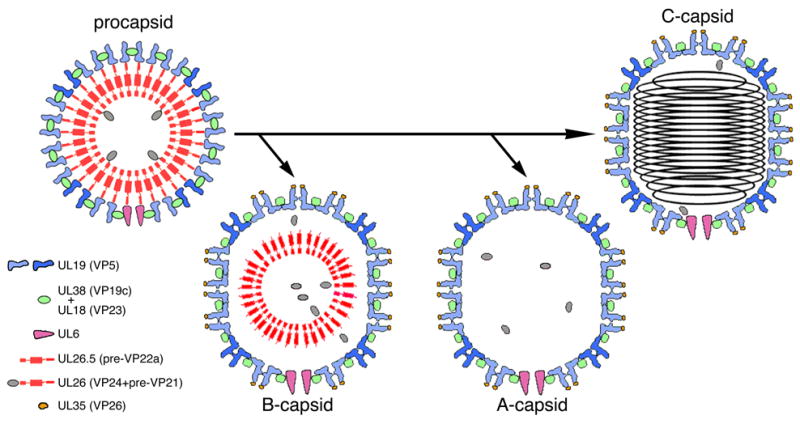
The procapsid surface shell is made up of hexamers (light blue subunits) and pentamers (mid-blue) of UL19, coordinated by triplexes, which are heterotrimers of UL18 and UL38. Its inner shell consists of the scaffolding proteins, UL26.5 and UL26, which is a fusion of the protease VP24 with VP21, an extended version of UL26.5. A single dodecamer of UL6 is present at the portal vertex. In maturation, the protease is released by autoproteolysis and processes the C-termini of the scaffolding proteins at the interface between the inner and outer shells. The surface shell then switches from round to polyhedral in shape, and UL35 binds to the outer tips of UL19 hexamers. The capsids are represented schematically in cross-sections that convey some but not all of their salient features.
