Fig. 2.
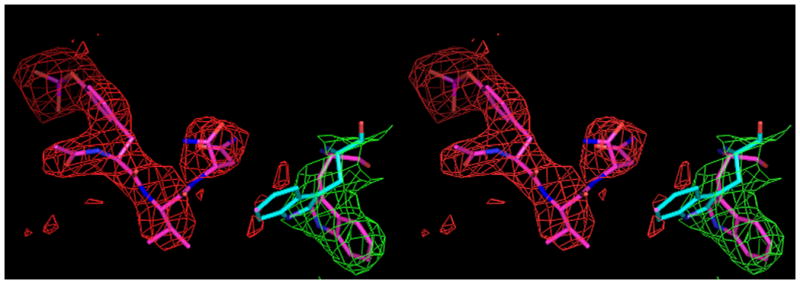
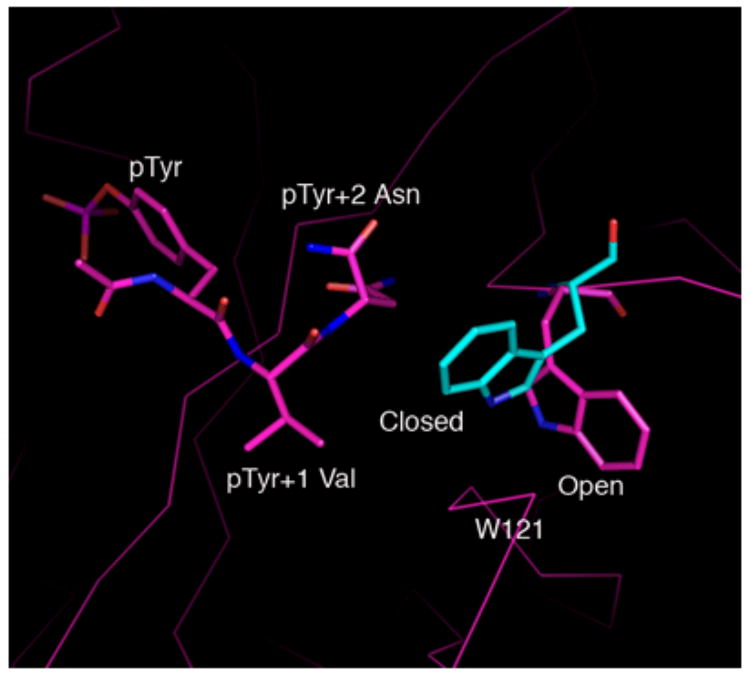
A, Shown in cross-eyed stereo are the 2Fo-Fc (green) and |Fo-Fc| (red) electron density maps contoured at 1.0 and 2.5 σ, respectively. The latter maps were calculated with tripeptide Ac–pYVN omitted. The |Fo-Fc| omit map clearly shows the location of the ligand despite the relatively low resolution data (3.2 Å). For information regarding the quality of electron density maps at this resolution see reference [37]. B, Open and closed conformations of W121. Shown is W121 from a monomeric Grb2-SH2/ligand complex (cyan) (PDB code 1JYR [14]) aligned to the Grb2-SH2 domain-swapped dimer/Ac–pYVN structure (magenta). In crystal structures of monomeric Grb2-SH2 W121 adopts the closed conformation (cyan), bringing the idole ring within close contact to the ligand pTyr+2 residue. W121 from the domain-swapped dimer/Ac–pYVN complex adopts the open conformation and points away from the ligand binding site (magenta). W121 also adopts the open conformation in uncomplexed domain-swapped dimer.
