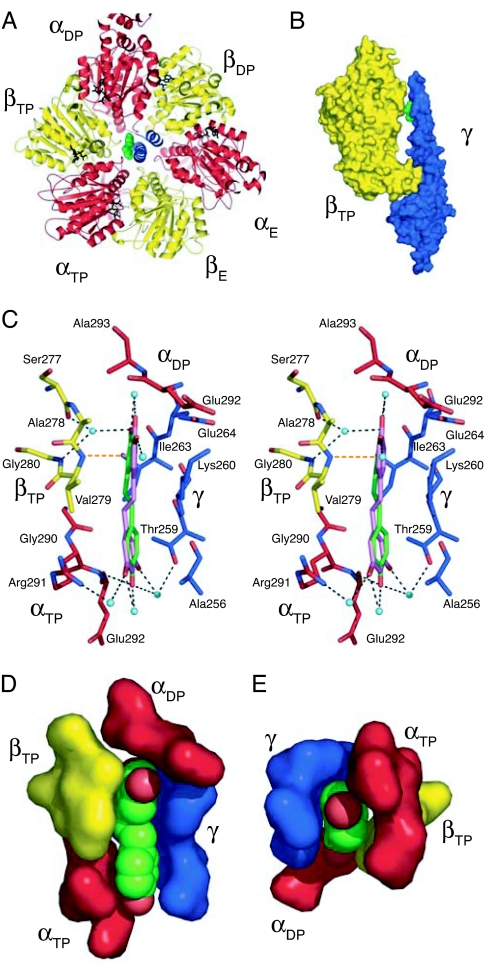
Fig. 2.
The site of binding of resveratrol in bovine F1-ATPase. The α-, β- and γ-subunits are red, yellow, and blue, respectively, and resveratrol is green. (A) Ribbon view of F1-ATPase upwards from the mitochondrial membrane along the central axis of the γ-subunit, showing the inhibitor in solid representation bound between the γ- and βTP-subunits. (B) Side view of a solid representation of resveratrol bound in a pocket in F1-ATPase between the γ- and βTP-subunits. For clarity, the βDP and βE subunits and the three α-subunits have been removed. The pocket is in the “bearing” consisting of the sleeve provided by the N-terminal regions of α- and β-subunits in the “crown” domain of F1-ATPase and the α-helical tip of the C-terminal region of the γ-subunit. (C) Side view in stereo showing interactions of resveratrol with side chains in the binding pocket shown in stick representation with oxygen and nitrogen atoms in binding-site residues in red and dark blue, respectively. The residues shown are either within 4 Å of the inhibitor and form hydrophobic interactions, or they are linked to it via hydrogen bond networks (dotted lines) involving water molecules (light blue spheres), and by a hydrogen bond from the amido group of Val-279 to the π-electrons of the m-dihydroxyphenyl moiety of resveratrol (orange dotted line). (D and E) view of the binding pocket and bound resveratrol (with red oxygen atoms) in solid representation. D is the same view as in C, and in E, bound resveratrol and its binding pocket are viewed along the axis of γ-subunit, upwards from the mitochondrial membrane.