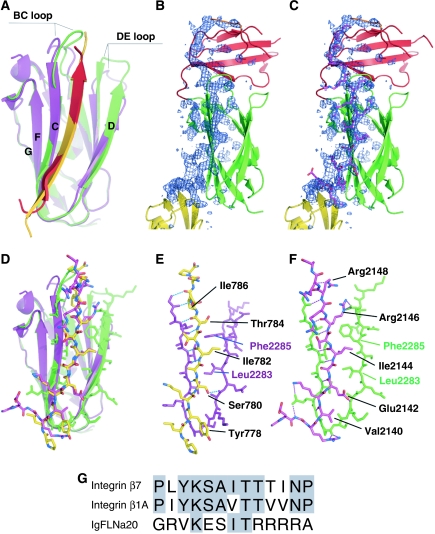
Figure 2.
Comparison of interaction of IgFLNa21 with IgFLNa20 and integrin β7. (A) Superimposition of the ribbon diagram of the interaction between the CD face of IgFLNa21 (green) and the first part of IgFLNa20 (red) with the published complex (2BRQ) between IgFLNa21 (purple) and the integrin β7 cytoplasmic domain (gold). The BC and DE loops that differ between the two IgFLNa21 structures are indicated. (B, C) Electron density corresponding to the first strand of IgFLNa20, shown as difference (omit) map calculated without the residues 2136–2159 (shown in purple in panel C). The map is shown as a blue mesh at 2.5σ. The side chains pointing toward β-strand D of IgFLNa21 are well visible in the electron density. (D) Similar superimposition as in panel A with the peptides and IgFLNa21 strand C and D shown as stick models. Colors as in panel A. (E, F) Details of interaction between IgFLNa21 (purple in panel E, green in panel F) and the integrin β7 peptide (E: yellow) and IgFLNa20 (F: red). In both cases, hydrogen bonds are shown as dashed lines. (G) Sequence alignment shows that the IgFLNa20 sequence has little similarity to integrin sequences. Notably the most conserved residues Lys (2141 in IgFLNa20) and Thr (2145) point out from the interaction surface.