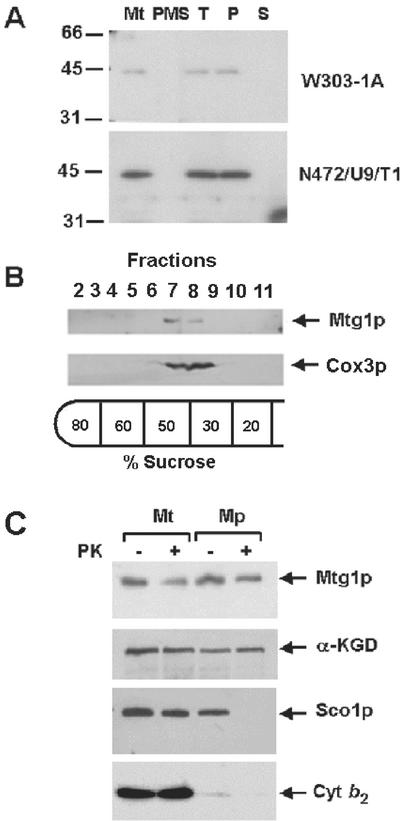
Figure 3.
Mitochondrial localization of Mtg1p. (A) Mitochondria and the postmitochondrial supernatant fractions were prepared from the wild-type W303-1A and from N472/U8/T1, the mtg1 point mutant transformed with MTG1 on a high-copy plasmid. A sample of mitochondria was sonically irradiated and centrifuged at 100,000 × gav for 30 min. The pellet, consisting of submitochondrial particles, was suspended in the starting volume of buffer. Equivalent volumes of mitochondria (Mt), sonicated mitochondria (T), submitochondrial particles (P), and the supernatant obtained after centrifugation of the sonicated mitochondria (S) were separated on a 12% polyacrylamide gel. The amount of mitochondrial and postmitochondrial supernatant proteins (PMS) loaded on the gel was 40 μg. Proteins were transferred to nitrocellulose paper, and the Western blot was treated with antiserum against Mtg1p followed by a second incubation with 125I-labeled protein A (Schmidt et al., 1984). The antibody-antigen complexes were visualized by exposure of the blot to Kodak x-ray film. (B) Mitochondria of wild-type yeast, at a protein concentration of 10 mg/ml, were converted to submitochondrial particles by sonic disruption as in A. After centrifugation, the particles were resuspended in the starting volume of 10 mM Tris-Cl, pH 7.5, and 0.5 ml was layered on 5 ml of a discontinuous sucrose gradient prepared in 10 mM Tris-Cl, pH 7.5. The gradient was centrifuged at 260,000 × gav for 2 h. The gradient was fractionated into 12 equal fractions. Fractions 2 through 11 were separated on a 12% polyacrylamide gel, transfer to nitrocellulose, and treated with antibodies against cytochrome oxidase subunit 3 (Cox3p) and Mtg1p. Proteins were visualized with anti-rabbit IgG peroxidase-conjugated secondary antibody (Sigma, St. Louis, MO) using the Super Signal chemiluminescent substrate kit (Pierce, Rockford, IL). (C) Mitochondria were prepared by the method of Glick and Pon (1995) from W303-1A and the overexpressor N472/U8/T1. The mitochondria were suspended at a protein concentration of 8 mg/ml in 0.6 M sorbitol, 20 mM HEPES, pH 7.5. To prepare mitoplasts (Mp) the mitochondrial suspension was diluted with 8 volumes of 20 mM HEPES, pH 7.5. For controls, mitochondria (Mt) were diluted with 8 volumes of 0.6 M sorbitol, 20 mM HEPES, pH 7.5. Proteinase K (prot K) was added to one half of each sample at a final concentration of 100 μg/ml and incubated for 60 min on ice. The reaction was stopped by addition of phenylmethylsulfonyl fluoride to a final concentration of 2 mM and the mitochondria and mitoplasts were recovered by centrifugation at 100,000 × gav. The pellets were suspended in, 0.6 M sorbitol, 20 mM HEPES, pH 7.5, and proteins were precipitated by addition of 0.1 volume of 50% trichloroacetic acid and heated for 10 min at 65°C. Mitochondrial and mitoplast proteins from wild-type (80 μg) and the transformant (40 μg) were separated by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with antibody against Mtg1p, Sco1p, α-ketoglutarate dehydrogenase (αKGD), and cytochrome b2 (Cyt b2). Antibody-antigen complexes were visualized as in B.