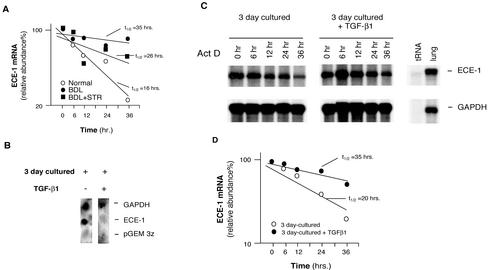
Figure 8.
Transcriptional and posttranscriptional effects of TGF-β1 in stellate cell ECE-1 mRNA during liver injury and hepatic wound healing. (A) Stellate cells from normal livers, those from injured livers (BDL for 8 d), or those from injured livers exposed to the soluble TGF-β receptor were isolated and allowed to adhere to culture vessels for 48 h. Actinomycin D (10 μg/ml) was added to the medium and total cellular RNA was isolated at the indicated times. ECE-1 mRNA was detected by RNase protection assay; specific ECE-1 bands were normalized to the signal for GAPDH, and the data used to determine ECE-1 mRNA half-life t1/2 by interpolation as described previously (Ross, 1995) (n = 3). (B) Stellate cells were isolated from normal rats and were allowed to initiate their activation cascade (by culture in the presence of serum containing medium for 3 d, a model that mimics in vivo activation of stellate cells). Culture medium with or without of TGF-β1 (5 ng/ml) was introduced for 24 h and nuclei were isolated. Nuclear transcription assay was performed as described in MATERIALS AND METHODS. (C) Isolated stellate cells (as in B) were used; culture medium with or without TGF-β1 (5 ng/ml) was introduced for 24 h and then actinomycin D (10 μg/ml) was added. Total cellular RNA was isolated at the indicated times. RNA (10 μg/sample) was hybridized with 32P-labeled cRNAs (ECE-1 and GAPDH) as described in MATERIALS AND METHODS and an RNase protection assay is shown. (D) Specific ECE-1 bands were scanned, quantitated, and normalized to the signal for GAPDH, and the data are presented graphically.