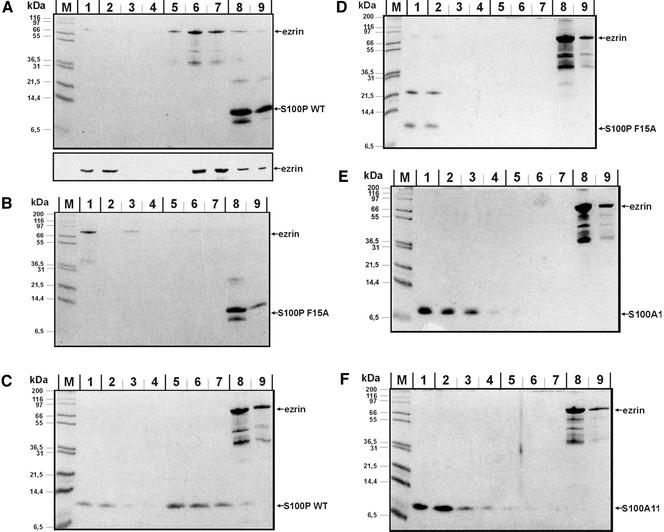
Figure 2.
Direct interaction between ezrin and S100P derivatives analyzed by affinity chromatography approaches. In each experiment, His-tagged protein (WT S100P, F15A S100P, or ezrin) was bound to the Ni-matrix, and the potential ligand (ezrin, WT S100P, F15A S100P, S100A1, or S100A11) was added as purified untagged protein in the fluid phase. Binding reactions were carried out in the presence of Ca2+ and columns were then washed with a Ca2+-containing buffer. Subsequently the columns were treated with an EGTA-containing buffer to elute Ca2+-dependently bound proteins, and finally they were stripped with imidazole-containing buffer. Equivalent amounts of all fractions were subjected to SDS-PAGE, and proteins were visualized by Coomassie staining. To unambiguously identify the recombinantly expressed ezrin, samples of an independent experiment shown in the bottom part of panel A were analyzed by immunoblotting with antiezrin antibodies. Lanes 1 always show the flow-through, lanes 2–4 washing steps in the presence of Ca2+, lanes 5–7 EGTA elution steps, and lanes 8 and 9 fractions of the imidazole stripping, respectively. M, molecular weight markers. (A) Immobilized His-WT S100P with thrombin-cleaved ezrin in the fluid phase; (B) immobilized His-F15A S100P with thrombin-cleaved ezrin in the fluid phase; (C) immobilized His-ezrin with thrombin-cleaved WT S100P in the fluid phase; (D) immobilized His-ezrin with thrombin-cleaved F15A S100P in the fluid phase; (E) immobilized His-ezrin with untagged S100A1 in the fluid phase; (F) immobilized His-ezrin with untagged S100A11 in the fluid phase.