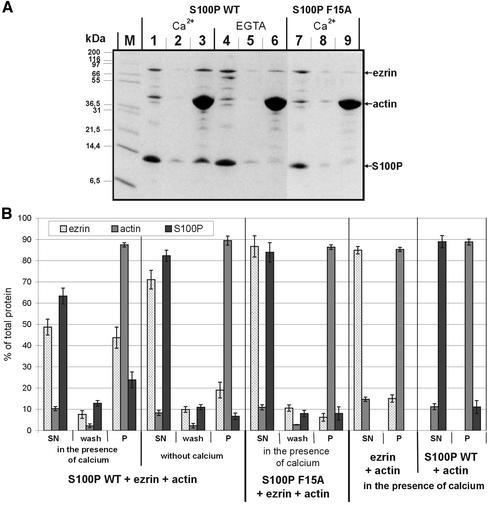
Figure 4.
F-actin cosedimentation assays. (A) WT S100P (lanes 1–6) or F15A S100P (lanes 7–9) were mixed with ezrin and F-actin either in the presence of Ca2+ (lanes 1–3 for WT S100P, and lanes 7–9 for F15A S100P) or in the presence of EGTA (lanes 4–6). After incubation and high-speed centrifugation the resulting supernatants were collected (lanes 1, 4, and 7). The remaining pellets were washed once with incubation buffer (lanes 2, 5, and 8 represent the supernatants of these washes), and the final pellets (lanes 3, 6, and 9) were dissolved by boiling in SDS-sample buffer. Equivalent aliquots of all fractions were subjected to SDS-PAGE. M, molecular weight markers. (B) Quantification of the cosedimentation data. Coomassie-stained gels containing the different fractions of F-actin cosedimentation experiments (one example is shown in A) were analyzed on a Lumi-Imager (Boehringer Mannheim) for quantification of the stained protein bands. For each protein, the signal obtained was normalized to the total amount of the respective protein added to reaction, which was set arbitrarily to 100%. Error bars indicate SD of the eight independent experiments analyzed. SN is the supernatant after the first centrifugation, i.e., the protein not bound to or incorporated into F-actin filaments, wash is the supernatant after washing of the F-actin pellet in incubation buffer, and P represents the protein fraction remaining in the final pellet, i.e., the proteins incorporated into (actin) or bound to (ezrin, S100P) F-actin filaments. Note the F-actin-ezrin cosedimentation in the presence of WT but not F15A S100P, which is only observed in the presence of Ca2+. The amount of ezrin copelleted in the absence of Ca2+ (EGTA, lane 6) is not increased with increasing actin concentrations (in contrast to reactions performed in the presence of Ca2+, unpublished results) and thus most likely represents a nonspecific background. Cosedimentation experiments performed only with ezrin and F-actin reveal no appreciable ezrin pelleting, indicating that the ezrin preparation used is in the dormant conformation and not activated by mild proteolysis (B).