Scheme 2.
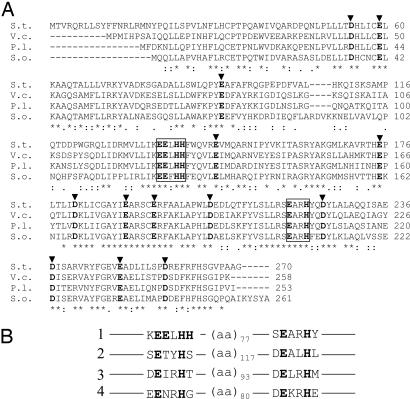
Multiple sequence alignment of MiaE proteins. (A) Amino acid alignments of MiaE proteins from S. typhimurium (S.t, Q08015) and putative tRNA ms2io6A hydroxylases from Vibrio cholerae (V.c, Q9KQT8); Photorhabdus luminescens subsp. Laumondii (P.l, Q7MB84) and Shewanella oneidensis (S.o, Q8CX43). The alignments were performed with the ClustalW program. Totally conserved amino acid residues are indicated by asterisks. The conserved EXXH motifs are framed. Black arrowheads indicate amino acid residues that could be potential ligands for diiron center. The numbers refer to amino acid residues for S. typhimurium protein. (B) Primary sequence homologies of diiron carboxylate proteins. 1, MiaE from S. typhimurium (Q08015); 2, R2 subunit of ribonucleotide reductase from E. coli (P69924); 3, hydroxylase component of methane mono oxygenase from Methylosinus trichosporium (P27353); 4, stearoyl carrier Δ9-desaturase protein from Ricinus communis (P22337).