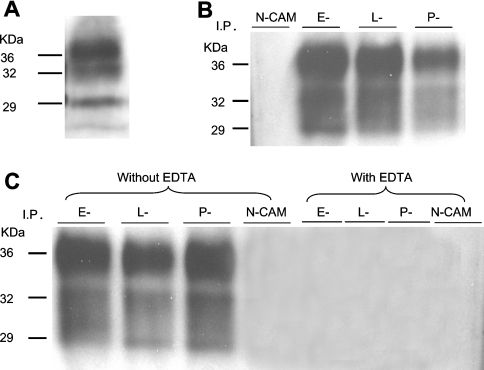
Figure 6. Human brain PrPC binds selectins and binding is Ca2+-dependent.
(A) Proteins in normal human brain homogenate were separated by SDS/PAGE and then immunoblotted with anti-PrPC mAb, 7A12. It showed the typical three bands at approx. 29, 32 and 36 kDa. (B) Proteins in normal human brain homogenates were precipitated with NCAM–Ig, E-selectin–Ig, L-selectin–Ig or P-selectin–Ig fusion protein. Precipitated proteins were then separated by SDS/PAGE and immunoblotted with anti-PrPC mAb, 7A12. All three selectins but not the NCAM–Ig fusion protein precipitated three proteins with apparent molecular masses of 29, 32 and 36 kDa, a pattern identical with the one seen in (A). (C) When precipitation with selectin–Ig was carried out in the presence of 10 mM EDTA, no PrP can be precipitated by selectin–Ig fusion proteins. Therefore binding of all three selectin–Ig fusion proteins to brain-derived PrPC is also calcium-dependent. I. P., immunoprecipitated.