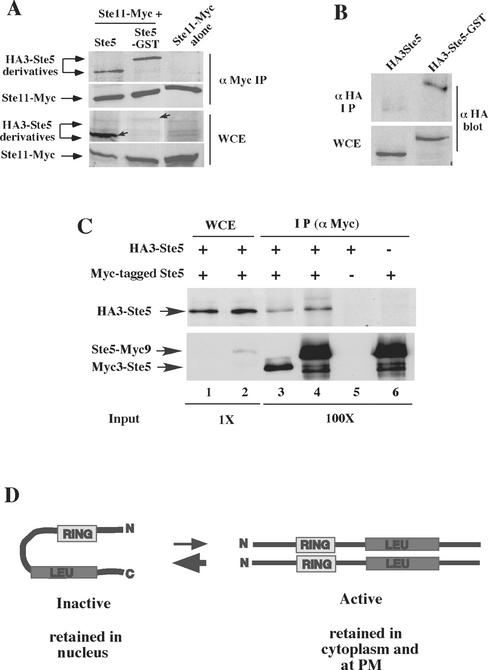
Figure 8.
Ste5-GST associates with more Ste11 and has a more accessible N terminus. (A) Ste5-GST associates with more Ste11. Ste11-Myc was immunoprecipitated from EY1775 cells expressing Ste11-Myc (pNC245) with either HA3-Ste5 (pSKM87) or HA3-Ste5-GST (pYMY74). Strains were grown in 2% galactose medium for 5 h to induce the expression of Ste11-Myc. (B) GST enhances the accessibility of the N terminus of Ste5 to antibody. Immunoblot of the relative level of HA3-Ste5 and HA3-Ste5-GST in whole cell extracts and α-HA (12CA5) immunoprecipitates. Strain EY1775 (ste5Δ) expressed either HA3-Ste5 (pSKM87) or HA3-Ste5-GST (pYMW74). (C) Ste5 oligomerizes at very low levels. Coimmunoprecipitation of Myc- and HA-tagged derivatives of Ste5 was done using extracts from ste5Δ (EY1775) cells cotransformed with HA3-Ste5 (pSKM87) and either Ste5-Myc9 (pSKM19) or Myc3-Ste5 (pYMW138). The left side (WCE) shows the amount of HA3-Ste5, Ste5-Myc9, and Myc3-Ste5 in 4.5 μg of whole cell extract. The right side (IP) shows the amount that is immunoprecipitated with 9E10 from 450 μg of WCE. At this exposure, Myc3-Ste5 is not detected in the WCE, whereas Ste5-Myc9 is detected due to six extra copies of Myc. (D) Model depicting Ste5 undergoing a conformational change from an inactive monomer to an active oligomer. The Ste5 monomer is shown in an autoinhibitory conformation that protects the RING-H2 domain and Ste11 binding site. The oligomer is shown in a more open conformation with more accessible RING-H2 domain and Ste11 binding site.