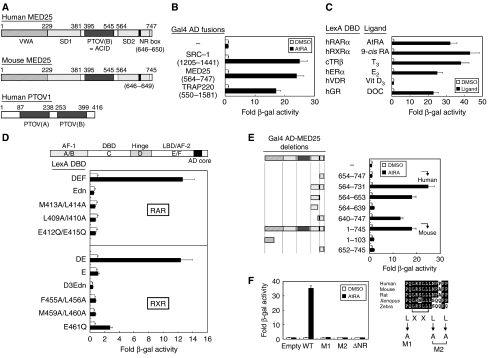
Figure 1.
Characterization of the interaction between MED25 and RAR in yeast. (A) Structural features of MED25 (human and mouse homologs) and PTOV1 (human). Notable domains include a von Willebrand factor type A (VWA) domain, a synapsin I domain 1 (SD1), a PTOV (a conserved region found in prostate tumor overexpressed protein 1) domain, a VP16 activator-interacting domain (ACID), an SD2 domain, and an NR box (LRSLL). (B) Isolation of MED25, which interacts with RAR DEF in the presence of retinoid, by yeast two-hybrid screening. Interactions between other known RAR cofactors (SRC-1 and TRAP220) and RAR are shown for comparison. (C) Interaction of MED25 with other nuclear receptors. The assays were performed using the indicated NRs in the presence of their cognate ligands. (D) The AF-2 AD core of RAR/RXR is critical for its ligand-dependent interaction with MED25. The location of the AF-2 AD core is shown (Edn, E region with deleted AF-2 AD, residues 212–403 of hRARα; D3Edn, D3E region with deleted AF-2 AD, residues 237–448 of mRXRα), and the point mutations are indicated. (E) Mapping of the MED25 domain responsible for the interaction with RAR. Additional deletions were constructed using the original human MED25 (residues 564–747) clone isolated by yeast-two hybrid screening. For full-length MED25, the mouse homolog was used. Schematic representations of full-length MED25 and its deletion mutants. (F) The NR box (LRSLL) of MED25 is critical for the interaction. Four NR box mutants (L → A) were designed on the basis of an alignment of MED25 from various mammalian species, as indicated. In all experiments, yeast two-hybrid and β-galactosidase assays were used to determine the interaction between LexA-fused nuclear receptors, including RAR, and Gal4 AD-fused RAR cofactors, including MED25 (and its mutants). Fold activity indicates the value relative to that of the Gal4 AD empty control.