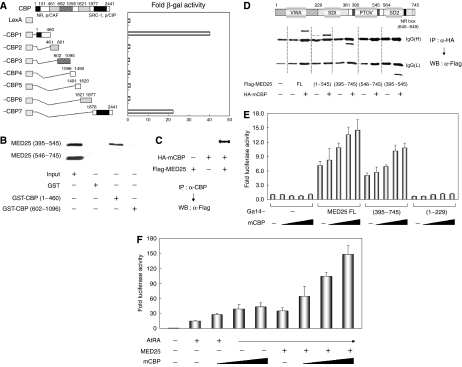
Figure 6.
Cooperation of MED25 with CBP. (A) The interaction between MED25 and CBP in yeast. Yeast two-hybrid assays were performed using LexA DBD fused to CBP deletions and Gal4 AD fused to MED25. Interactions were monitored by β-galactosidase assay. (B) In vitro interactions between MED25 and CBP. [35S]-labeled MED25 (residues 395–545) or MED25 (residues 546–745) was mixed with GST, GST-CBP (residues 1–460), or GST-CBP (residues 602–1096). The GST pull-down results were visualized by autoradiography. (C) Interaction between MED25 and CBP in mammalian cells. HA-tagged CBP and Flag-tagged MED25 expression vectors were transfected alone or together into NIH3T3 cells. IP of the cellular extracts with anti-CBP antiserum was followed by WB with anti-Flag antibody. (D) Mapping of the MED25 domain needed to bind CBP. NIH3T3 cells were transfected with HA-tagged CBP and Flag-tagged MED25 (or its deletions). WB using anti-Flag antibody was performed using precipitates obtained from cellular extracts and anti-HA antibody. The locations of the IgG heavy and light chains are indicated. (E) Effect of CBP on the intrinsic transcriptional activity of MED25. NIH3T3 cells were transfected with Gal4 DBD-responsive luciferase reporter, 0.1 μg of Gal4 DBD-fused MED25 (or its deletions), and increasing amounts (0, 0.01, 0.05, 0.1, and 0.2 μg) of CBP expression vector. (F) Effect of CBP on MED25-mediated transcriptional enhancement of RAR. NIH3T3 cells were transfected with RARE-tk-luciferase reporter, RARα, and increasing amounts (0, 0.1, 0.2, and 0.4 μg) of CBP expression vector in the absence or presence of MED25. Extracts were subjected to luciferase assays as described (E, F).