Figure 1.
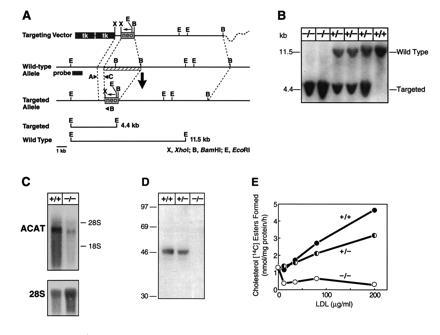
Generation of Acact−/− mice. (A) Targeting vector strategy. Homologous recombination of the vector results in the interruption of a 5′ early exon (amino acids 50–100) of ACAT by the neo gene (at amino acid 63 of 540) and causes a ≈2-kb deletion of genomic sequences (striped bar). Targeted clones were identified by a unique 4.4-kb EcoRI restriction fragment as detected by a ≈1-kb HindIII–SacI probe located upstream of the vector or by PCR analysis. Targeting frequency was 1 in 268. (B) Southern blot demonstrating Acact−/− mice. Of 226 offspring from heterozygous matings, 66 were wild-type mice, 104 were heterozygotes, and 56 were homozygotes (P = 0.573 by χ2 analysis versus the expected 1:2:1 Mendelian distribution). (C) Northern blot of preputial gland RNA in Acact−/− mice. Blots were probed with a mouse ACAT cDNA fragment and reprobed with a primer specific for 28S RNA to control for RNA sample loading. Reverse transcription–PCR analysis (not shown) revealed that the shorter ACAT mRNA in Acact−/− mice was caused by exon skipping around the inserted exon containing the neo mutation and the following exon, which was deleted in the targeting vector. The skipping of these two exons generates a 211-bp deletion and a frameshift resulting in a premature stop codon. (D) Immunoblot demonstrating lack of ACAT protein in preputial gland homogenates from Acact−/− mice. Each lane contains 100 μg of protein; membranes were incubated with DM10 (19). As a control, the same homogenates were tested for the LDL receptor–related protein, which was detectable at approximately equivalent amounts in each sample (not shown). (E) Cholesterol esterification activity in embryonic fibroblasts in response to LDL. Pulse assays were performed as described (21) on fibroblasts derived from Acact+/+, Acact+/−, or Acact−/− embryos. The experiment was repeated three times; data from a representative experiment are shown.
