Figure 1.
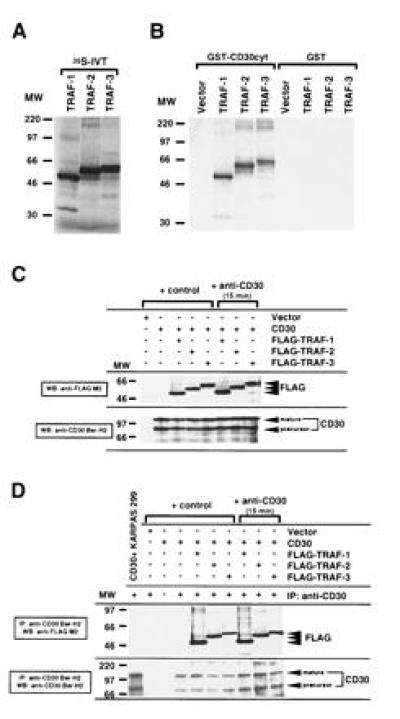
Association of human TRAF-1, TRAF-2, and TRAF-3 with the cytoplasmic domain of CD30. (A) TRAF-1, -2, and -3 proteins were [35S]methionine-labeled by in vitro translation (IVT). Ten microliters (20%) of each in vitro translation reaction was loaded. (B) The cytoplasmic domain of CD30 was fused to GST (GST–CD30cyt) and bound to glutathione beads. GST or GST–CD30cyt were incubated with 25% of the in vitro-translated TRAF proteins. The fraction bound to glutathione beads was analyzed by SDS/PAGE. Comparable GST loading was confirmed by staining with amino black and Western blotting against GST (data not shown). More than 90% of TRAF-1 and TRAF-2 and 50–60% of TRAF-3 respectively bound to GST–CD30cyt. (C) Western blot analysis of CD30 and FLAG expression in transfected 293 cells. 293 cells were transiently cotransfected with the full-length CD30 expression vector (5 μg) and the indicated FLAG-tagged TRAF constructs (5 μg). After 48 hr, lysates were prepared from unstimulated and anti-CD30-stimulated (15 min) transfected 293 cells. Thirty microliters of lysates (1/30) were separated by SDS/PAGE, blotted, preblocked, and probed for CD30 and/or FLAG-tagged TRAF proteins using anti-CD30 Ber-H2 or anti-FLAG M2 mAb followed by horseradish peroxidase-conjugated goat anti-mouse IgG and detection using enhanced chemiluminescence (FLAG indicates the transfected FLAG-tagged TRAF protein). The molecular weight (MW) standard is shown (in thousands). (D) In vivo interaction of TRAF-1, -2, and -3 with the cytoplasmic domain of CD30. One hundred microliters of the cell lysates analyzed in C were subjected to immunoprecipitation with the anti-CD30 Ber-H2 mAb. This mAb shows no cross-reactivity with the transfected TRAF proteins (data not shown). Coprecipitating FLAG-tagged TRAF proteins were detected by immunoblotting using the anti-FLAG M2 mAb (indicated by FLAG). KARPAS 299 is a large cell anaplastic lymphoma line.
