Figure 2.
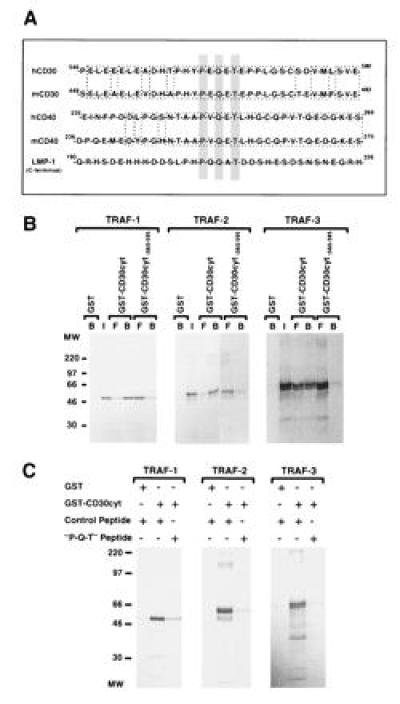
A critical CD30 receptor sequence motif involved in TRAF binding. (A) A common sequence motif is involved in TRAF binding and is shared by CD30, CD40, and LMP1. Sequence aligment of the cytoplasmic domains of CD30, CD40, and LMP1 show the TRAF binding motif centered around the consensus sequence PXQXT (gray box). The mouse and human CD30 and CD40 sequences are highly conserved around the putative TRAF binding motif (1, 42, 43, 44). (B) The distal region of the cytoplasmic domain of CD30 is involved in TRAF binding. GST or GST-fusion proteins containing the full-length cytoplasmic domain of CD30 (CD30cyt) or a carboxyl-terminal deletion (CD30 lacking the carboxyl-terminal 36 aa; CD30cytΔ560-595) bound to glutathione beads were incubated with in vitro-translated TRAF-1, -2, and -3 proteins (25%), respectively. Reaction mixtures were analyzed by SDS/PAGE and autoradiography. A proximal deletion mutant of the cytoplasmic domain of CD30 (CD30cytΔ408-430) showed similar interaction as the full-length GST–CD30cyt fusion protein with all three TRAF proteins (data not shown). Western blotting confirmed that equal amounts of GST were used (data not shown). I, input; B, bound; and F, free. (C) Interaction of TRAF proteins with the cytoplasmic domain of CD30 was blocked by a peptide overlapping the TRAF binding domain in CD30. [35S]Methionine-labeled TRAF-1, -2, and -3 proteins (25%) were preincubated with 50-fold excess of a random control peptide (PSTMVYDACRMIRERIPEA) or the “P-Q-T” peptide encompassing amino acids 556–570 of the cytoplasmic domain of CD30 (HTPHYPEQETEPPLG) for 2 hr at 4°C, followed by incubation with the full-length cytoplasmic domain of CD30 fused to GST or GST alone for 1 hr at 4°C and analyzed by SDS/PAGE. Equal GST protein loading was confirmed by Western blotting (data not shown).
