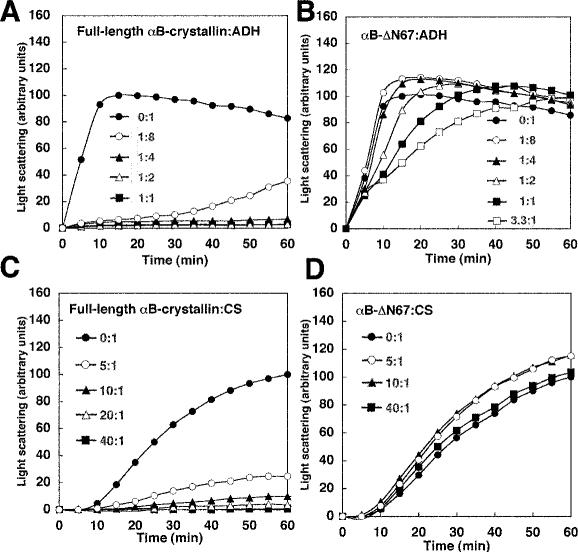
Fig 4.
Chaperone activities of deletion mutant αB-ΔN67 and full-length αB-crystallin for ADH and CS. Kinetics of thermal aggregation with 20 μM ADH at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (A) or αB-ΔN67 (B) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), 10 μM (open triangle), 20 μM (closed square), and 66 μM (open square). These assays were performed in a buffer of 50 mM sodium phosphate, 150 mM NaCl (pH 7.0), and 2 mM EDTA. Kinetics of thermal aggregation of 0.5 μM CS at 42°C alone (closed circle) or in the presence of full-length αB-crystallin (C) or αB-ΔN67 (D) at concentrations of 2.5 μM (open circle), 5 μM (closed triangle), 10 μM (open triangle), and 20 μM (closed square). These assays were performed in 40 mM HEPES (pH 7.5). In each case, protein aggregation was determined as in Figure 3