Abstract
Xenobiotic cannabinoid CB1/CB2-receptor agonists appear to possess broad-spectrum antiemetic activity since they prevent vomiting produced by a variety of emetic stimuli including the chemotherapeutic agent cisplatin, serotonin 5-HT3-receptor agonists, dopamine D2/D3-receptor agonists and morphine, via the stimulation of CB1-receptors. The purpose of this study was to evaluate whether structurally diverse cannabinoids [Δ9-THC, (delta -9- tetrahydrocannabinol); (Δ8-THC, delta -8-etrahydrocannabinol); WIN55,212-2, (R (+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)), methyl] pyrolol [1, 2, 3 – de] – 1, 4 benzoxazinyl] – (1-naphthalenyl) methenone mesylate); and CP55,940, ((-) - 3- [2-hydroxy-4- (1,1 - dimethylheptyl] - 4 – [3 – hydroxypropyl] cyclohexan –1 – ol)), can prevent radiation-induced emesis. Exposure to total body radiation (0, 5, 7.5 and 10 Gy) caused robust emesis in the least shrew (Cryptotis parva) in a dose-dependent manner (ED50= 5.99 (5.77 – 6.23) Gy) and all animals vomited at the highest tested dose of radiation. In addition, the radiation exposure reduced locomotor behaviors to a significant but mild degree in a non-dose-dependent fashion up to one hour posttreatment. Radiation-induced emesis (10 Gy) was blocked in a dose-dependent manner by the CB1/CB2– receptor agonists with the following ID50potency order: CP55,940 (0.11 (0.09 - 0.12) mg/kg) > WIN55, 212,2 (3.65 (3.15 – 4.23) mg/kg) = Δ8-THC (4.36 (3.05 – 6.22) mg/kg) > Δ 9-THC (6.76 (5.22 – 8.75) mg/kg). Although the greater antiemetic potency and efficacy of Δ8-THC relative to its isomer Δ9-THC is unusual as the latter cannabinoid possesses higher affinity and potency for cannabinoid receptors in functional assays, the current data support the results of a clinical study in children suggestive of complete protection from emesis by Δ8-THC. This effect has not been clinically observed for Δ9-THC in cancer patients receiving chemo- or radiation-therapy.Cannabinoids prevented the induced emesis via the stimulation of cannabinoid CB1- receptors because the CB1(SR141716A) – and not the CB2(SR144528) –receptor antagonist reversed both the observed reduction in emesis frequency and shrew emesis protection afforded by either Δ9-THC or CP55,940 against radiation-induced emesis. These findings further suggest that the least shrew can be utilized as a versatile and inexpensive small animal model to rapidly screen the efficacy of investigational antiemetics for the prevention of radiation-induced emesis.
Keywords: radiation; vomit; least shrew; Δ9-THC; Δ8-THC; WIN55,212,2; CP55; 940; SR141716A; SR144528; cannabinoid; CB1-receptor; CB2-receptor
1. Introduction
Chemotherapy, radiotherapy and surgery are the main modalities of cancer treatment. In contrast to chemotherapy, radiotherapy and surgery are generally for local treatment. Vomiting and nausea are among the most distressing aspects of cancer treatment (Griffin et al., 1996; Kayl and Meyers, 2006), which impair patients’ quality of life (Osoba et al., 1996). Numerous published clinical trials as well as reviews have dealt with the problems, possible solutions and mechanisms by which highly emetogenic chemotherapeutics such as cisplatin induce nausea and vomiting (e.g. Hesketh et al., 2003; Veyratt-Follet et al., 1997). Although not all patients tend to vomit following radiotherapy, a significant proportion do experience emesis following total body or upper abdominal irradiation (Maranzano, 2001). Effective control of radiation-induced emesis is important since emesis persisting throughout radiotherapy can cause dehydration, electrolyte imbalance and malnutrition (Jereczek-Fossa et al., 2001).
Relatively few randomized clinical trials have evaluated the efficacy of various antiemetic drugs preventing radiation-induced emesis (Maranzano, 2001; Feyer et al., 2005; Scarantino et al., 1994). Dopamine D2-receptor antagonists are effective in only about 50% of patients, whereas serotonin 5HT3-receptor antagonists, either alone or in combination with a glucocorticoid such as dexamethasone, appear to be more, but not completely, effective antiemetics against highly emetogenic regimens such as total body irradiation. A small number of available clinical studies also suggest that cannabinoids (e.g. delta-9-tetrahydrocannabinol, nabilone or levonantradol) can be useful for the prevention of radiation-induced emesis (Ungerleider et al., 1982; Priestman et al., 1987, Lucraft and Palmer, 1982). The D2- and 5-HT3- receptor antagonists’ antiemetic efficacy have been suggested to be dependent upon selective blockade of corresponding specific dopaminergic- and serotonergic-receptor subtypes (Maranzano et al., 2005). However, the receptor mechanism by which cannabinoid agonists prevent radiation-induced emesis remains unknown. Four chemically diverse groups of cannabinoid receptor agonists exist (Pertwee, 1999). Delta-9-tetrahydrocannabinol (Δ9-THC) and its isomer Δ8- THC are members of the “classical cannabinoids”, whereas CP55,940 ([1α, 2β-(R)- 5α] – (-)-5-(1,1-dimethyl)-2-[5-hydroxy-2 (3-hydroxypropyl) cyclohexyl-phenol]) represents the nonclassical cannabinoid group. The aminoalkylindole cannabinoids consist of the pravadoline derivatives such as WIN55,212-2 [R (+) -[2,3-dihydro-5-methyl-3- [(morpholinyl)methyl] pyrolol [1, 2, 3 – de]-1, 4-benzoxazinyl] – (1-naphthalenyl) methanone mesylate].The fourth group are endocannabinoids and these constitute arachidonic acid derivatives such as anandamide and 2-arachidonoylglycerol. Exogenous cannabinoids (plant derived and synthetic) and endocannabinoids produce their effects mainly via the stimulation of at least two cannabinoid receptors called CB1and CB2(Onaivi et al., 2006)The cannabinoid CB1receptor is preferentially located in the brain, spinal cord and peripheral neurons, whereas the cannabinoid CB2receptor is mainly found on peripheral tissues. The clinical potential of antiemetic cannabinoids predates basic animal investigations (Darmani, 2002a). Recent animal studies have shown that cannabinoid CB1- and CB2- receptor agonists of diverse structure and activity prevent chemotherapy-induced emesis via the activation of cannabinoid CB1receptors (Darmani, 2001a;2001b; Darmani et al., 2003; Van Sickle et al., 2001; 2005; Kwiatkowska et al, 2004). Indeed, the cannabinoid CB1- receptor is present in several emetic nuclei in the brainstem (Darmani et al., 2003; Van Sickle et al., 2003). A more recent study also indicates expression of CB2receptor messenger RNA and its protein in such nuclei and suggests a possible modulatory role for CB2receptors in emesis (Van Sickle et al.,2005).
Preliminary studies indicated that exposure to radiation induces emesis in the least shrew (Cryptotis parva). This animal model has been extensively used in our laboratory for the characterization of emetic circuits (reviews: Darmani, 2002a and 2006). The purpose of this investigation was to evaluate: 1) whether radiation-induced emesis is dose-related in the least shrew, 2) the antiemetic structure activity-relationship among the cited diverse cannabinoid CB1/CB2receptor agonists against radiation-induced emesis, 3) whether the antiemetic activity of cannabinoids against radiation-induced emesis is a CB1- or CB2- receptor-mediated event, 4) if radiation-exposure can alter motor behaviors in the least shrew since such treatment causes lethargy in patients (Jones et al., 2005) and can reduce locomotion in rodents (Van der Meeren and Lebaron-Jacobs, 2001) and nonrodent emetic species (King and Landauer, 1990).
2. Materials and methods
2.1. Animals and drugs
Shrews (C. parva) were used as test animals and were bred and maintained in our animal facilities. Both male and female shrews (4-5g, 45-60 days old) were used only once in the current study. The feeding and maintenance of shrews are fully described elsewhere (Darmani, 1998; Darmani et al., 1999). Delta-9-tetrahydrocannabinol (Δ9 THC), its isomer Δ8THC, and WIN55,212-2 [R (+) - [2, 3 - dihydro -5-methyl - 3 - [morpholinyl) methyl] pyrolol [1, 2, 3 - de] - 1, 4 -benzoxazinyl] - (1 - naphthalenyl) methanone mesylate] were obtained from Sigma/RBI (St. Louis, MO). SR141716A [N-piperidino-5-(4-chlorophenyl) -1-(2, 4 -di-chlorophenyl) – 4-methylpyrazole-3-carboxamide], and SR144528 [N-(1S)-endo-1, 3, 3-trimethylbicyclo [2.2.1] heptan-2-yl] 5-(4-chloro-3-methylphenyl) -1- (4-methylbenzyl)-1-pyrazole-3-carboxamide], were generously donated by Sanofi Recherché (Montpellier, France). CP55,940 [(-) - 3 - [2-hydroxy -4 - (1, 1 – dimethylheptyl] -4 - [3-hydroxypropyl] cyclohexan -1-ol] was obtained from Pfizer, Inc. (Groton, CT). Cannabinoid agonists and antagonists were initially dissolved to twice the stated concentrations in a 1:1:18 solution of ethanol: emulphor: 0.9% saline. The drug concentrations were then diluted by the addition of an equal volume of saline. All drugs were administered at a volume of 0.1 ml/10g of body weight. All animals received care according to the Guide for the Care and Use of Laboratory Animals, DHSS Publication, revised, 1985.
2.2. Radiation exposure
On the test day, the shrews were transported from the animal quarters of the Kirksville College of Osteopathic Medicine (Kirksville, MO) to the George Rea Cancer Treatment center in large covered cages to minimize exposure to the outside environment. Animal transportation took less than 2 min since the cited buildings are less than 100 meters apart. The shrews were allowed to acclimate to the test environment by placing two animals per cage (20 × 18 × 21 cm clean clear plastic cages) for one hour prior to radiation exposure. Thirty min prior to radiation treatment each pair of shrews were offered 4 meal worms (Tenebrio sp.). A shrew holder was constructed from a slab of plastic containing two parallel rectangular grooves (each 3 × 1 × 1 cm) in the middle of the plain of the slab (Fig.1). The two shrews were individually placed into the two grooves and with a coverslip plastic slab the shrews were kept in place. Once placed in the plastic slab, the shrews were unable to move or rotate. The construction of the entire holder was such that the distance between the dorsal plain of the shrews and the upper surface of the slab was equal to the distance between the lower surface of the slab and the ventral plain of the animals. To allow the shrews to breathe and to reduce buildup of heat during the radiation procedure each groove had three small holes along its length and one hole along its width (Fig.1).
Fig. 1.
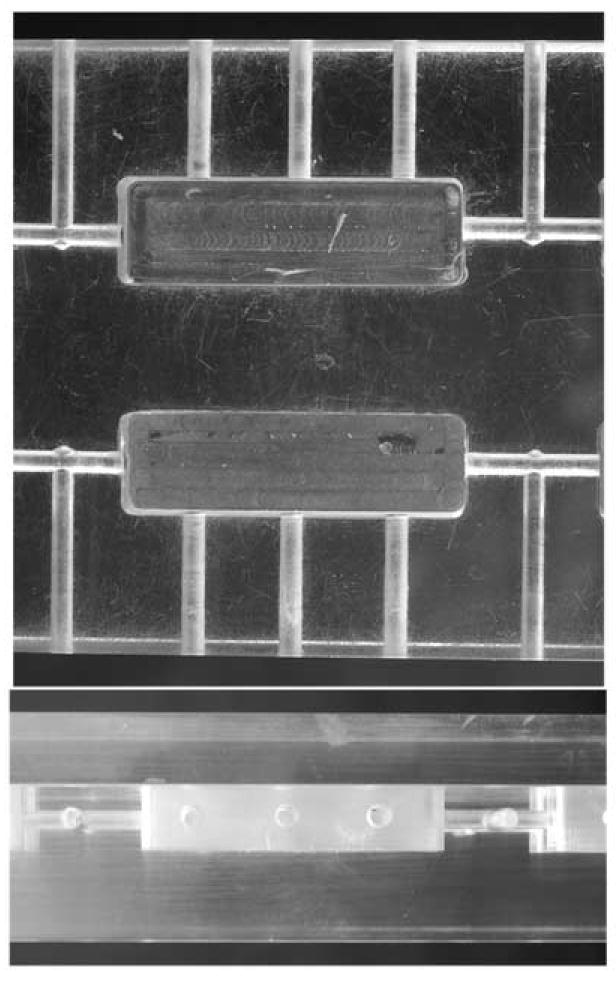
This figure shows the top (upper photograph) and the side (bottom photograph) views of the plastic shrew holder. The top view shows two parallel rectangular bed grooves (each 3×1×1 cm) in the middle of the plain of the plastic slab. Each shrew bed has 3 small holes drilled along its length and one hole along its width. The side view shows locations of drilled holes and coverslip as well as the position of bed groves relative to its upper and lower surfaces.
At the designated time the plastic shrew holder containing two shrews was placed under the exposure field of a Varian Clinac 600 C/D linear accelerator. The shrew holder was situated in a source-to-skin distance of 100 cm with an exposure field set to 40 × 40 cm. The shrews were exposed to doses of 0, 5, 7.5 and 10 Gy (Gray). To minimize any differences due to shrew orientation in the holder, half of the radiation exposure was initially given, and immediately the shrew holder was then inverted, and the remaining dose was delivered. The exposure procedure took approximately 3 min. The control animals were placed in the shrew holder and underwent identical events except they did not receive radiation.
2.3. Emesis studies
The present protocols were based upon our preliminary studies as well as published findings in the least shrew (Darmani, 2001b; Darmani et al., 2003). Our preliminary 90 min observation studies had shown that all bouts of vomiting occurred within 30 min of exposure to the cited doses of radiation. Thus, to evaluate whether radiation exposure can induce emesis, different groups of shrews were given varying doses of radiation (0, 5, 7.5 and 10 Gy, n = 6 shrews per group). Immediately following radiation, each shrew was placed in the observation cage and the frequency of vomiting (oral ejections of food or liquid; mean ± S.E.M.) was recorded for each individual shrew by a trained observer for the next 30 min. These data showed that 10 Gy radiation produces a maximal and robust frequency of emesis in all exposed shrews. This dose of radiation was chosen for subsequent drug interaction studies to determine the antiemetic potential of the selective cannabinoid CB1/CB2-receptor agonists (Δ9 -THC, Δ8 -THC,WIN55,212-2 and CP55,940).
Based upon our existing extensive data against a wide variety of emetic stimuli, 4-5 different doses of each cannabinoid CB1/CB2 receptor agonists were employed for radiation-induced emesis inhibition studies. Thus, at 0 time shrews were allowed to acclimate to the test environment for 30 min and were given 4 meal worms. At 30 min different doses of either Δ9 – THC [(0, 1, 5, 10 or 20 mg/kg, i.p., n = 8-12 shrews per group); Δ8 -THC(0,1,2.5, 5 or 10 mg/kg, i.p., n = 8 shrews per group]; WIN55,212-2 (0, 1, 2.5, 5 or 10 mg/kg, i.p., n = 12 shrews per group) or CP55,940 (0, 0.025, 0.01 and 0.3 mg/kg, i.p., n = 12 shrews per group); were administered to different groups of shrews. At 60 min each shrew was exposed to 10 Gy radiation and emesis was recorded for 30 min immediately following the radiation exposure.
To demonstrate whether the antiemetic effects of cannabinoids is a cannabinoid CB1receptor – mediated event, nonemetic subcutaneous (s.c.) doses of either the cannabinoid CB1-receptor antagonist SR141716A (0, 1, 5 and 10 mg/kg, n = 8-10 shrews per group) or the cannabinoid CB2-receptor antagonist SR144528 (0 and 10 mg/kg, n = 8 per dose), were used to attempt to reverse the antiemetic activity of two (Δ9 -THC and CP55,940) of the cited cannabinoid CB1/CB2 receptor agonists against radiation-induced emesis. For this experiment, a fully effective antiemetic dose of either Δ9 -THC (20 mg/kg. i.p.) or CP55,940 (0.3 mg/kg, i.p.) was chosen. Thus, at 0 time, different groups of shrews were injected either with vehicle, or the cited doses of SR141716A or SR144528, and were then offered four mealworms. Thirty min later, the corresponding treated shrews received either Δ9- THC or CP55,940. At 60 min shrews were exposed to 10 Gy radiation and the emesis frequency was recorded for the next thirty min as described earlier.
Since large doses of SR141716A (>10 mg/kg, i.p. or > 40 mg/kg, s.c.) by itself produce emesis in the least shrew (Darmani, 2001c), the additive or synergistic emetic actions of various doses of SR141716A (0, 1, 5 and 10 mg/kg, s.c., n = 8 shrews per group) were investigated with a single dose of radiation exposure (10 Gy). SR141716A was administered 60 min prior to radiation and the frequency of emesis was recorded for 30 min post-radiation exposure as described earlier.
2.4. Locomotor studies
Shrews were exposed to 0, 5, 7.5 and 10 Gy (n = 8 per group) radiation as described earlier. Following treatment, shrews were transported back from the George Rea Cancer Treatment Center to the behavioral lab in less than 3 min in covered cages. At 5 min each shrew was individually placed in an observation cage (28 × 28 × 14 cm.) and locomotor parameters were recorded for the next 10 min by a computerized video tracking, motion analysis and behavior recognition system [EthoVision (version 2.0) Noldus Information Technology, Costerweg, Netherlands]. The parameters of EthoVision were set to record the following locomotor activities: 1) spontaneous locomotor activity in terms of the total distance moved in meters, and 2) the rearing frequency [a rearing event is recorded as a 20% decrease in surface area when shrews stand upright as seen by the overhead video camera (Darmani, 2001b)]. Since the 10 Gy dose reduced both motor parameters, the prolonged effects of this radiation dose (0 and 10 Gy, n= 8 per group) were also investigated for 10 min intervals at 50 and 110 min post-radiation exposure in different groups of shrews.
2.5. Statistical analysis
The data on the frequency of emesis were analyzed by the Kruskal-Wallis (KW) nonparametric one-way analysis of variance and post hoc analysis by Dunn’s multiple comparisons test. A P value of < 0.05 was necessary to attain statistical significance. The incidence of emesis (number of shrews vomiting) was analyzed by the χ2 exact test to determine whether there were differences between groups. When appropriate, pairwise comparisons between vehicle treated-controls and drug treated groups were made by the Fisher’s exact test. For some emesis data, the two-tailed Mann-Whitney test was used. The ED50 (the radiation dose that induced emesis in 50% of shrews) and ID 50 values (the inhibitory dose that prevented emesis in 50% of shrews) were calculated by the use of a computerized program (Prism, San Diego, CA) via linear regression using sigmoidal dose-response with variable slope and constraints respectively set at: 1) 0 for 0 Gy and 100% for 10 Gy for radiation dose-response studies, and 2) 0 for bottom (0mg/kg) and 100% for top dose utilized for vomit inhibition studies. Depending on the analyses a one-way or two-way analysis of variance (ANOVA) followed by either Dunnett’s or Bonferroni’s multiple comparison tests were used to analyze the locomotor activity data.
3. Results
3.1. Emesis Studies
Total body radiation exposure (0, 5, 7.5 and 10 Gy) caused dose-dependent increases in the frequency of vomiting (KW 3, 28 = 15.23, P < 0.02). Dunn’s multiple comparisons posthoc test showed that relative to the sham-exposed control group, a significant increase (P < 0.01) in the mean (± SEM) emesis frequency occurred at the 10 Gy dose (Fig. 2A). The χ2exact test showed that the percentage of shrews vomiting in response to radiation also increased in a dose-dependent fashion with an ED50of 5.99 (5.77 – 6.23, and R2 = 1) Gy (χ2 3, 28 = 13.3, P < 0.003) (Fig. 2B). Fisher’s exact posthoc test showed that the 5 (P < 0.03) and the 10 Gy doses (P < caused significant potentiations in the number of shrews vomiting. The 10 Gy dose was chosen for vomit induction in subsequent drug interaction studies since it caused emesis in all tested animals in the initial dose-response studies. However, in subsequent drug-interaction studies, 93 -100% of vehicle-pretreated shrews vomited in response to 10 Gy radiation.
Fig. 2.
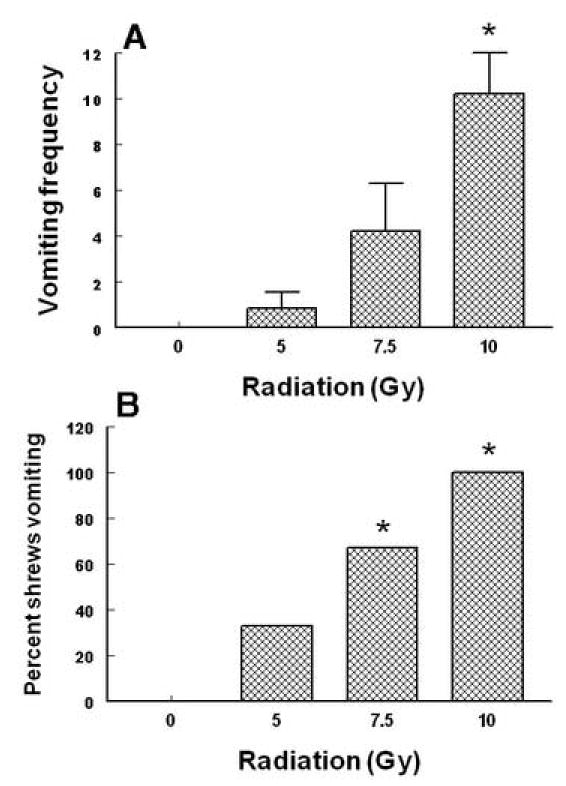
This figure represents the emetic dose-response effects of total body radiation during the 30 min post exposure observation period in the least shrew. Graph A depicts enhancements in the frequency of emesis (mean ± S.E.M.) whereas graph B shows percentage of shrews vomiting. *Significantly different from sham-treated control at P < 0.05.
Intraperitoneally administered Δ9 –THC (0, 1, 5, 10 and 20 mg/kg) attenuated the mean vomit frequency [1.7 (P > 0.05), 74.1 (P > 0.05), 74.1 (P > 0.05) and 92.1% (P < 0.01), respectively] caused by radiation in a dose-dependent manner (KW 4, 39 = 19.85, P < 0.0005) (Fig. 3A). Δ9 –THC also protected shrews from emesis [4.6 (P > 0.05), 59.1 (P< 0.018), 31.8 (P> 0.05) and 86.4% (P < 0.03), respectively] with an ID 50 of 6.76 (5.22 – 8.75, and R2 = 0.8) mg/kg (χ2 4, 39 = 16.94, P < 0.001 ) (Fig. 3B). The isomer of Δ9 –THC, Δ8 –THC (0, 2.5, 5 and 10 mg/kg),appeared to be relatively more effective in reducing the mean emesis frequency since it completely and dose-dependently blocked vomiting (KW 3, 28 = 16.3, P < 0.001) (Fig. 4A) and a significant effect was seen at its 10 mg/kg dose (P < 0.01). Moreover, Δ8 –THC dose-dependently protected shrews from vomiting at the 5 (50%, P< 0.04) and 10 mg/kg doses (100%, P < 0.0001) with an ID 50 of 4.36 (3.05 – 6.22, and R2 = 0.8) mg/kg (χ23, 28 = P < 0.00005)(Fig.4B). The pravadoline analog of Δ9 –THC, WIN55, 212-2 (0, 1, 2.5, 5 and 10 mg/kg), also relatively potently and significantly attenuated the frequency of induced-vomiting at the 5 (91.4, P < 0.01) and 10 mg/kg (93.2%, P < 0.01) doses (KW 4, 55 = 28.43, P < 0.0001) (Fig. 5A). Moreover, significant reductions in the number of shrews vomiting (63.7, P< 0.005 and 81.4%, P < 0.0003) occurred at its 5 and 10 mg/kg doses with an ID50of 3.65 (3.15 – 4.23, and R2 = 0.9) mg/kg (χ2 4, 55 = 22.6 P < 0.00007) (Fig. 5B). The nonclassical cannabinoid, CP55,940 (0, 0.025, 0.1 and 0.3 mg/kg), potently reduced the frequency of induced emesis in a dose-dependent fashion (KW 3, 44 = 15.91, P < 0.0012) and a significant reduction was observed at its 0.3 mg/kg dose (96.4%, P < 0.01) (Fig. 6A). Likewise, it significantly protected shrews from vomiting at 0.3 mg/kg dose (81.5%, P < 0.01) with an ID50of 0.11 (0.09 – 0.12, and R2 = 0.8) mg/kg (χ2 3, 44 = 15.6 P < 0.001) (Fig. 6B).
Fig. 3.
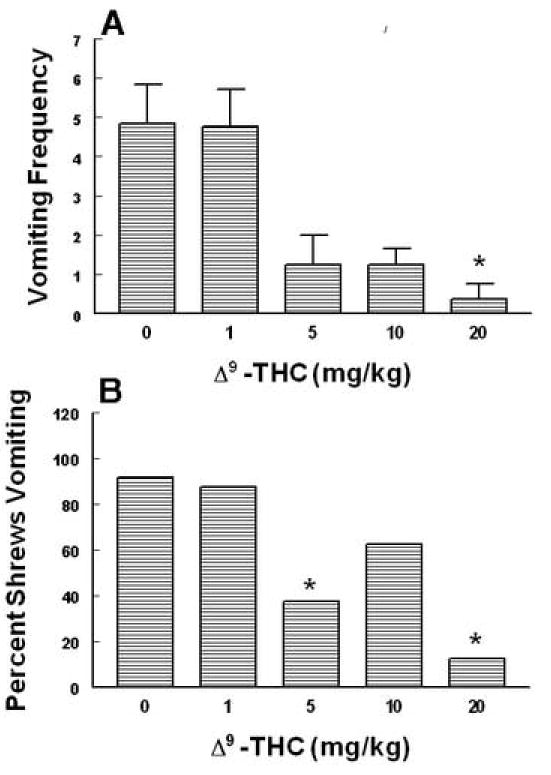
The antiemetic effects of Δ9 – THC against radiation-induced emesis in the least shrew. Different groups of shrews received i.p. either vehicle (0 mg/kg) or the cited doses of Δ9 – THC 30 min prior to a 10 Gy radiation exposure. Emetic parameters were recorded for 30 min postradiation. Graph A depicts attenuation in the frequency (mean ± S.E.M.) of emesis whereas graph B shows reduction in the percentage of shrews vomiting. *Significantly different from vehicle control at P < 0.05.
Fig. 4.
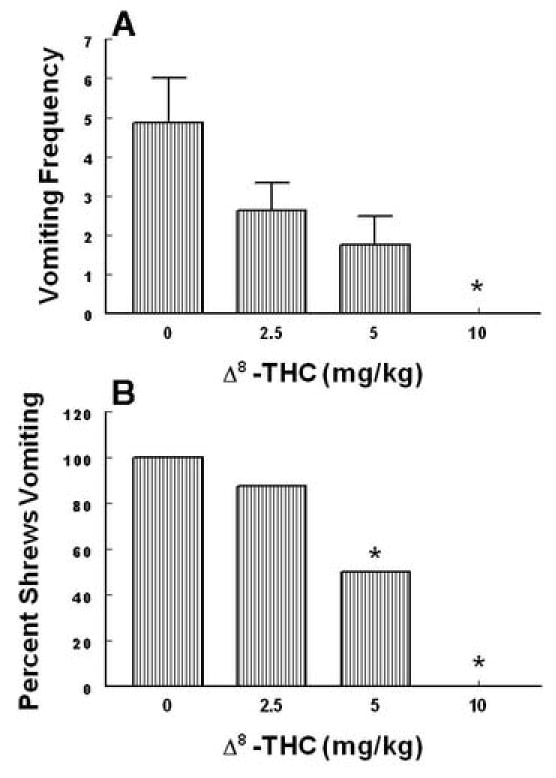
The antiemetic effects of Δ8– THC against radiation-induced emesis in the least shrew. Different groups of shrews received i.p. either vehicle (0 mg/kg) or the cited doses of Δ8 – THC 30 min prior to a 10 Gy radiation exposure. Emetic parameters were recorded for 30 min postradiation. Graph A depicts attenuation in the frequency (mean ± S.E.M.) of emesis whereas graph B shows reduction in the percentage of shrews vomiting. *Significantly different from vehicle control at P < 0.05.
Fig. 5.
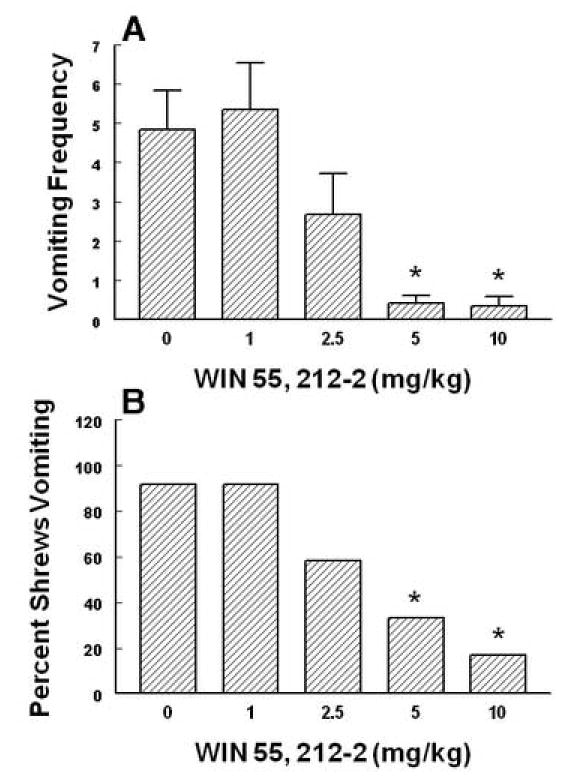
The antiemetic effects of WIN55,212-2 against radiation-induced emesis in the least shrew. Different groups of shrews received i.p. either vehicle (0 mg/kg) or the cited doses of WIN55,212-2 30 min prior to a 10 Gy radiation exposure. Emetic parameters were recorded for 30 min postradiation. Graph A depicts attenuation in the frequency (mean ± S.E.M.) of emesis whereas graph B shows reduction in the percentage of shrews vomiting. *Significantly different from vehicle control at P < 0.05.
Fig. 6.
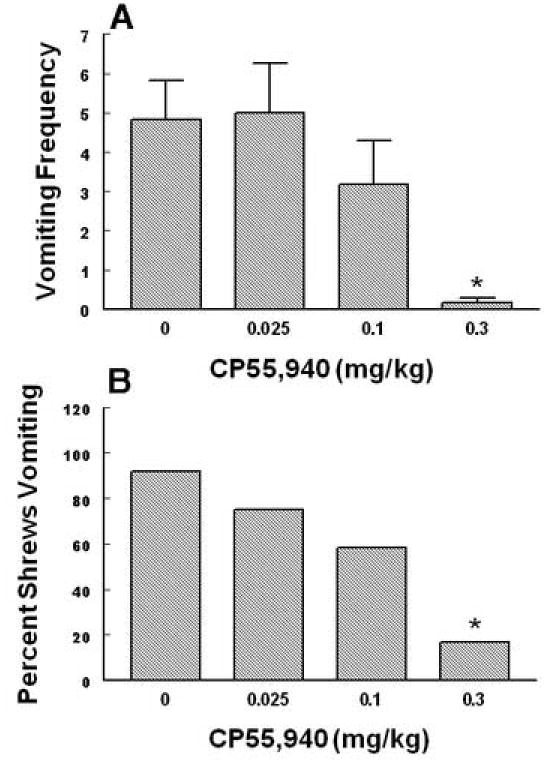
The antiemetic effects of CP55,940 against radiation-induced emesis in the least shrew. Different groups of shrews received i.p. either vehicle (0 mg/kg) or the cited doses of CP55,940 30 min prior to a 10 Gy radiation exposure. Emetic parameters were recorded for 30 min postradiation. Graph A depicts attenuation in the frequency (mean ± S.E.M.) of emesis whereas graph B shows reduction in the percentage of shrews vomiting. *Significantly different from vehicle control at P < 0.05.
To demonstrate whether the antiemetic effect of the discussed cannabinoids is mediated via the cannabinoid CB1, or CB2or both receptors, reversal of the antiemetic capacity of an effective dose of two of the cannabinoid agonists (Δ9 –THC, 20 mg/kg i.p. or CP55,940, 0.3 mg/kg i.p.) against radiation-induced emesis was investigated by various doses of the cannabinoid CB1-receptor antagonist, SR141716A (0, 1, 5 and 10 mg/kg , s.c.), or the cannabinoid CB2- receptor antagonist SR144528 (0, 10 mg/kg, S.C). SR141716A pretreatment reversed in a dose-dependent manner, both the observed reductions in emesis frequency [(KW 3,30 = 14.2, P < 0.003, Fig. 7B) and (KW 3, 30 = 14.1, P < 0.01, Fig. 7A ] and shrew emesis protection [(χ23, 30 = 12.1, P < 0.006) and (χ2 3, 30 = 11.8, P < 0.007) (Fig. 7B)] afforded respectively by Δ9 –THC and CP55, 940. In all cases significant reversals (P < 0.03) were seen at the 10 or 5 mg/kg doses of SR141716A. On the other hand, the 10 mg/kg dose of the cannabinoid CB 2 receptor antagonist, SR144528, failed to induce a significant reversal in any of the antiemetic effects of either Δ9 –THC or CP55,940 (data not shown).
Fig. 7.
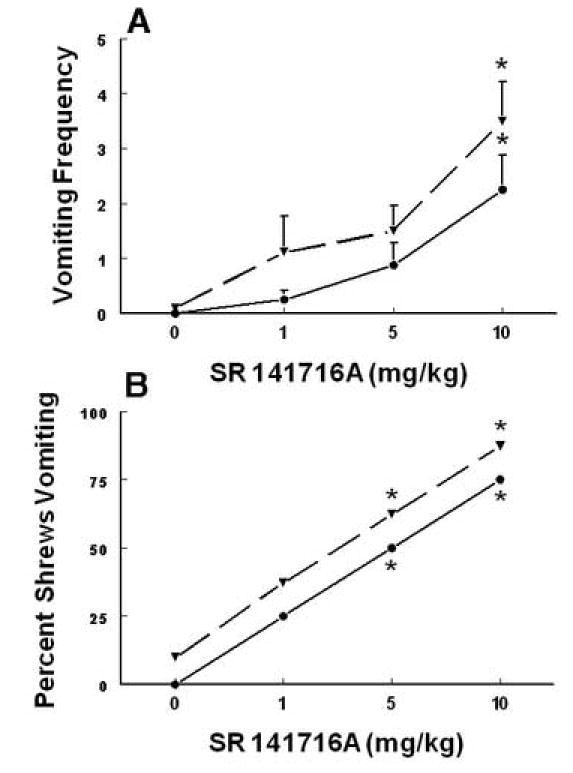
The ability of the cannabinoid CB1 receptor antagonist SR141716A (0, 1, 5 and 10 mg/kg, s.c.) to reverse the antiemetic effects of a fully effective dose of either Δ9 –THC (20 mg/kg, s.c., solid line) or CP55,940 (0.3 mg/kg, s.c., dashed line) to protect shrews from radiation (10 Gy) - induced emesis. SR141716A reversed the induced reductions in emesis frequency (graph A) and blocked the ability of both Δ9 –THC and CP55,940 to protect shrews from vomiting caused by radiation exposure (graph B). At 0 time, different groups of shrews received either vehicle or the cited doses of SR141716A s.c. and at 30 min Δ9 –THC or CP55,940. At 60 min shrews were exposed to 10 Gy radiation and emesis parameters were recorded for 30 min following the radiation exposure. *Significantly different from corresponding control at P < 0.05.
As discussed earlier, since intraperitoneal (> 10 mg/kg) and subcutaneous (> 40 mg/kg) administration of large doses of SR1141716A by itself causes emesis (Darmani, 2001c), the possible additive or synergistic emetic actions of SR141716A (0,1, 5 and 10 mg/kg) were investigated with a single dose of radiation (10 Gy) exposure. None of the tested doses had a significant effect either on the frequency or number of animals vomiting in response to the 10 Gy radiation (Fig. 8A, B).
Fig. 8.
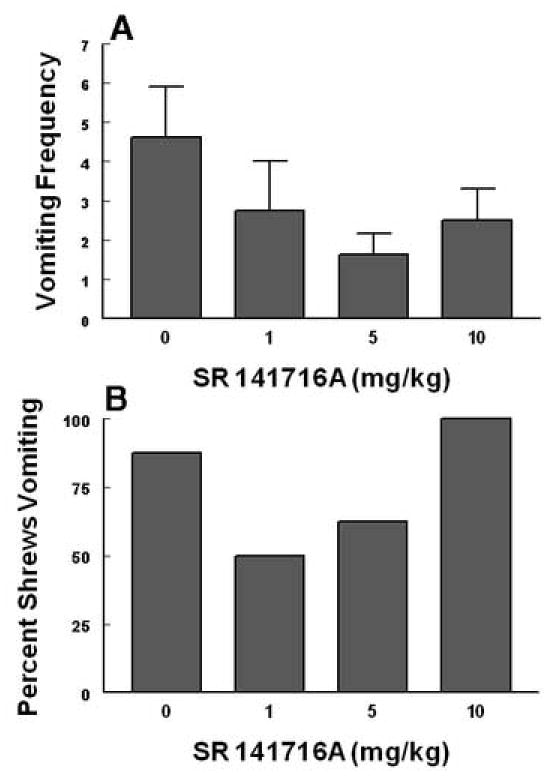
This figure indicates a lack of additive or synergistic emetic interactive effects of the cannabinoid CB1receptor antagonist SR141716A with a 10 Gy dose of radiation. The cited nonemetic s.c. doses of SR141716A were administered to different groups of shrews 60 min prior to a 10 Gy dose of radiation. Emetic parameters were recorded (graphs A and B) for 30 min after the last injection.
3.2. Locomotor activity studies
One way analysis of variance (ANOVA) indicated that total body exposure to varying doses of radiation (0, 5, 7.5 and 10 Gy) significantly attenuated the total distance moved (i.e. spontaneous locomotion) by shrews in the 10 min observation period starting from 5 min post-radiation exposure (F3, 28 = 4.2, P < 0.014). Dunnett’s multiple comparisons test showed that significant reductions were observed from the lowest tested radiation dose (44 (P < 0.05), 48 (P < 0.05) and 39% (P < 0.05) respectively) (Fig. 9A). Likewise, the frequency of rearing behavior was reduced by the radiation exposure (F3, 28 = 3.6, P < 0. 025). However, a significant reduction (41%, P< 0.05) was only observed at the highest tested dose (Fig. 9B).
Fig. 9.
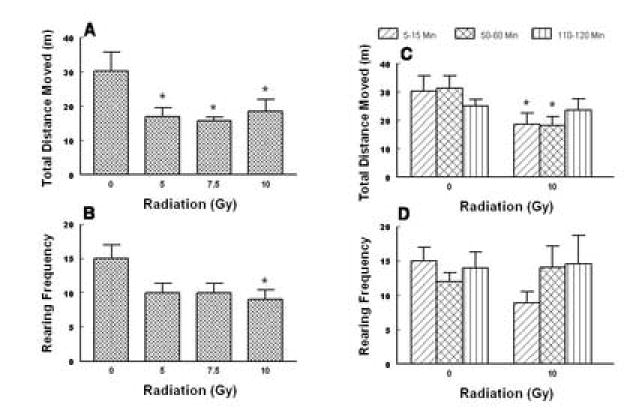
The inhibitory effects of the cited doses of total body radiation on both the spontaneous locomotor activity (total distance moved, mean ± S.E.M. in meters) and the frequency of rearing behavior (mean ± S.E.M.) in the least shrew. The motor behaviors were recorded for a 10 min observation period between 5-15 min (graphs A and B) or at 10 min observation periods during the 5-15, 50-60 and 110-120 min post-radiation exposure intervals (graphs C and D) on different groups of shrews. The motor behaviors were recorded by a computerized video tracking, motion analysis, and behavior recognition system (EthoVision). *Significantly different from sham-treated control group at P < 0.05.
Since the 10 Gy dose significantly reduced both locomotor parameters, the prolonged effects of this radiation dose and sham treatment were also investigated for 10 min intervals from the 50th and 110th min post-radiation exposure in different groups of shrews. Thus, two-way analysis of variance for dose and time exhibited significant differences for dose (F1, 42 = 8.64, P < 0.005) but not time (Figs. 9C, D). Fisher’s multiple comparisons posthoc test showed that relative to the corresponding sham-treated controls, the 10 Gy dose reduced the total distance moved by shrews during the 5-15 min (39%, P < 0.05) and 50-60 min (42%, P < 0.05) observation periods (Fig. 9C). However, a similar 2-way analysis of variance revealed no significant effect on the frequency of rearings exhibited by shrews following the radiation treatment (Fig. 9D).
4. Discussion
The present study introduces the least shrew as a new animal model of vomiting for radiation -induced emesis. To our knowledge this is the first report to investigate the antiemetic potential of cannabinoids against radiation-induced emesis in an animal model of vomiting. The least shrew appears to be a unique vomit model because: 1) this species produces emesis rapidly in a dose-and time-dependent manner (ED50= 5.99 (5.77 - 6.23) Gy) with a mean latency to first vomit between 28-12 min by the corresponding 5 and 10 Gy radiation doses. At the 10 Gy radiation dose all episodes of emesis occurred within 20 min of radiation exposure, 2) emesis occurs consistently with a maximal dose of 10 Gy from 93 – 100% of tested animals, and 3) due to its’ small size, relatively lower amounts of antiemetic test drugs are required and less effort is needed for cleaning the experimental apparatus since the amount of voided ingested food is very small. A decrease in the emetic efficacy of the 10 Gy radiation dose seem to occur in cannabinoid inhibition experiments since the mean vomit frequency was lower and not all shrews vomited in response to the radiation exposure. This is probably due to the amount of food present in the shrew stomach since in the original radiation dose-response experiments shrews were fed 30 min prior to radiation, whilst in the cannabinoid inhibition protocol animals received food 60 min prior to the 10 Gy exposure. Indeed, shrews have a high metabolic rate (Churchfield, 1990) and thus it is possible to assume that their gastric emptying rate would be rapid.
The ferret appears to be the most sensitive species to total body radiation since this species vomits with an ED50of 77 cGy and 100% incidence of emesis occurs at 201 cGy (King, 1988, 1990). However, ferrets tend to vomit for longer than 90 min post-radiation exposure. Dogs seem to be the next sensitive species while felines appear to be the least sensitive animals to vomit in response to radiation exposure (King, 1990). Humans, primates and least shrews react with similar sensitivity to acute radiation exposure and their emetic response lie between those of dogs and cats (King, 1990, present study). Although it cannot be equivocally stated that one species is more appropriate than another species as a model of emesis when studying a particular agent (King, 1990), the advantages of the least shrew in terms of the cost of examining the antiemetic potential of test drugs is self explanatory.
The attained results in the least shrew confirm the discussed clinical antiemetic potential (see introduction) of structurally diverse cannabinoid CB1/CB2 receptor agonists. However, Δ9–THC appears to be 3.3 times less effective against radiation-induced emesis (ED50= 5.99 mg/kg) than against cisplatin (20 mg/kg, i.p.) – induced vomiting (ED50= 1.8 mg/kg; Darmani, 2001a). Likewise, WIN55,212-2 is relatively less effective (5 times, Darmani, 2001b). Indeed, in both cases, the cited cannabinoids significantly and more effectively reduced cisplatin-induced emesis at lower doses. However, the cannabinoid antiemetic ID50potency order (CP55,940 > WIN55,212-2 > Δ9 –THC) against radiation-induced emesis is identical to that found for cisplatin in the least shrew (Darmani et al., 2003). One possible explanation for the discussed potency differences could be that cisplatin’s toxic effects develop relatively slowly via its metabolites (Daley-Yates and McBrien, 1984), while radiation’s toxic effects develop more rapidly. However, it is important to note that the 20 mg/kg dose of cisplatin is very toxic and within a week of exposure to such high doses most tested animals die across species (Sam et al., 2003; Preziosi et al., 1992, Costall et al., 1987), while all radiation-exposed shrews survived the 10 Gy radiation for many months. The more potent antiemetic efficacy of Δ8 –THC (ID50= (4.36 (3.05 – 6.22) mg/kg) relative to Δ9 – THC against radiation-induced emesis is surprising since this cannabinoid has lower affinity for CB1/CB2 receptors (Govaerts et al., 2004) as well as being less potent in suppression of motor behaviors (Darmani et al., 2001d) and in the induction of both psychoactivity in humans and tetrad of behaviors in rodents (Razdan, 1986). Albeit, a clinical study has shown that Δ8 – THC completely prevents chemotherapy-induced emesis in children (Abrahamov et al., 1995), where no such complete blockade of emesis at nonpsychoactive doses has been reported for Δ9 – THC in children or adult cancer patients treated with chemotherapeutics (see introduction).
It is well established that the discussed cannabinoids prevent emesis via the stimulation of cannabinoid CB1receptors when vomiting is induced by diverse emetic stimuli such as cisplatin (e.g. Darmani, 2001a,b; Darmani et al., 2003; Van Sickle et al., 2003; Kwiatkowska et al., 2004), morphine-6-glucuronide (Van Sickle et al., 2001), direct and indirect serotonin 5-HT3receptor agonists (Darmani and Johnson, 2004), dopaminergic D2/D3receptor agonists (Darmani and Crim, 2005; London et al., 1979), arachidonic acid and its precursor 2-arachidonoylglycerol (Darmani, 2002b). Indeed, in the latter studies, a cannabinoid CB1- and not a CB2- receptor antagonist, reversed the antiemetic efficacy of an effective dose of the tested cannabinoids in a dose-dependent fashion. In the current investigation, an effective antiemetic dose of two of the discussed cannabinoids (Δ9 – THC, 20 mg/kg and CP55,940, 0.3 mg/kg) were chosen for cannabinoid CB1 (SR141716A)- and CB2(SR144528)- receptor antagonist reversal study. SR141716A, but not SR144528, reversed the antiemetic efficacy of both Δ9 – THC and CP55,940. Thus, these findings suggest an important role for the cannabinoid CB 1 receptor in radiation-induced emesis. Although a modulatory role for the cannabinoid CB 2 receptor has been suggested against morphine-6-glucuronide – induced emesis (Van Sickle et al., 2005), cannabinoid CB2receptor agonists such as AM1241 and JWH133 had no antiemetic activity in the latter study. Moreover, as discussed earlier, various cannabinoid CB2 receptor antagonists are reported to be ineffective in reversing the antiemetic efficacy of different cannabinoid CB1/CB2receptor agonists against diverse emetic stimuli. Thus, if the cannabinoid CB2receptor has any antiemetic role in acute vomiting its antiemetic effect must be minor.
Radiotherapy in cancer patients causes fatigue and lethargy that is typically observed as part of the prodromal response to radiation (Jones et al., 2005). Likewise, radiation exposure suppresses locomotor behaviors in different animal species (Van der Meeren and Lebaron-Jacobs, 2001; King and Landauer, 1990). In the current study radiation exposure reduced both spontaneous locomotor activity and the frequency of rearing in the least shrew. However, the former motor parameter was not only significantly more sensitive to suppression at the lower doses of radiation but also the suppressive effects persisted up to one hour post-radiation, whereas the rearing behavior was only significantly reduced by the 10 Gy dose during the initial 5 – 10 min observation period. Furthermore, these motor suppressive effects were neither dose-dependent nor complete which seem to agree with clinical findings that some but not all patients suffer from severe lethargy that would interfere with daily work (Jones et al., 2005).
In summary, this study introduces the least shrew as a new animal model of radiation-induced emesis. Although radiation exposure significantly suppresses locomotor behaviors to a mild degree in a nondose-dependent manner, it concomittently does promote emesis in a dose-dependent fashion. The attained data suggest that structurally-diverse cannabinoid CB1/CB2receptor agonists possess broad-spectrum antiemetic activity, and as with other emetic stimuli,they seem to prevent radiation-induced vomiting via the stimulation of cannabinoid CB1receptor.
Acknowledgments
This work was supported by a grant from the National Cancer Institute (CA115331).The author thanks Mrs. Nona Williamson for typing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamov A, Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995;56:2097–2102. doi: 10.1016/0024-3205(95)00194-b. [DOI] [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ, Tattersal FD. Emesis induced by cisplatin in the ferret as a model for the detection of antiemetic drugs. Neuropharmacology. 1987;26:1321–1326. doi: 10.1016/0028-3908(87)90094-3. [DOI] [PubMed] [Google Scholar]
- Churchfield S. The Natural History of Shrews . Cornell Univ. Press Comstock Publishing Associates; Ithaca NY: 1990. [Google Scholar]
- Daley-Yates PT, McBrien DCH. Cisplatin metabolites in plasma, a study of their pharmacokinetics and importance in the nephrotoxic and antitumor activity of cisplatin. Biochem Pharmacol. 1984;33:3063–3070. doi: 10.1016/0006-2952(84)90610-5. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neurol Trans. 1998;105:1143–1154. doi: 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol Biochem Behav. 2001a;69:239–249. doi: 10.1016/s0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The cannabinoid antagonist/inverse agonist SR 141716A reverses the antiemetic and motor depressant action of WIN 55, 212-2 in the least shrew. Eur J Pharmacol. 2001b;430:49–58. doi: 10.1016/s0014-2999(01)01355-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Δ9 – Tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB 1 receptor anatagonist/inverse agonist SR141716A. Neuropsychopharmacology. 2001c;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT2A receptor-mediated behaviors in mice. Pharmacol Biochem Behav. 2001d;68:311–317. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Antiemetic actions of Δ9-tetrahydrocannabinol and synthetic cannabinoids in chemotherapy-induced nausea and vomiting . In: Onaivi ES, Sugiura T, DiMarzo V, editors. Biology of marijuana from gene to behavior. Taylor and Francis; London: 2002a. pp. 356 – 389. [Google Scholar]
- Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by Δ9-tetrahydrocannabinol and other cannabinoids. J Pharmacol Exp Therap. 2002b;300:34–42. doi: 10.1124/jpet.300.1.34. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Endocannabinoids and gastrointestinal function . In: Onaivi ES, Sugiura T, Di Marzo R, editors. Endocannabinoids. The brain and body’s marijuana and beyond . CRC/Taylor and Francis: Boca Raton ; 2006. pp. 395–419. [Google Scholar]
- Darmani NA, Crim JL. Delta-9-tetrahydrocannabinol prevents emesis more potently than enhanced locomotor activity produced by chemically diverse dopamine D2/D3receptor agonists in the least shrew (Cryptotis parva) Pharmacol Biochem Behav. 2005;80:35–44. doi: 10.1016/j.pbb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of Δ9-THC against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–212. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Sim-Selly LJ, Martin BR, Janoyan JJ, Crim JL, Parekh B, Breivogel CS. Antiemetic and motor depressive actions of CP55 940: Cannabinoid CB 1 receptor characterization, distribution and G-protein activation. Eur J Pharmacol. 2003;459:83–95. doi: 10.1016/s0014-2999(02)02815-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B. The role of D2and D3dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew) J Neurol Transm. 1999;106:1045–1061. doi: 10.1007/s007020050222. [DOI] [PubMed] [Google Scholar]
- Feyer PCh, Maranzano E, Molassiotis A, Clark-Snow RA, Roila F, Warr D, Olver I. Radiotherapy-induced nausea and vomiting (RINV): antiemetic guidelines. Support Care Cancer. 2005;13:122–128. doi: 10.1007/s00520-004-0705-3. [DOI] [PubMed] [Google Scholar]
- Govaerts SJ, Hermans E, Lambert DM. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharmac Sciences. 2004;23:233–243. doi: 10.1016/j.ejps.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersal MH. On the receiving end. V: patients perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol. 1996;7:189–195. doi: 10.1093/oxfordjournals.annonc.a010548. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van Belle S, Aapro M, Tattersal FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Pharmacol. 2003;39:1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy related fatigue: How to assess and how to treat the symptom. A commentary. Tumori. 2001;87:147–151. doi: 10.1177/030089160108700308. [DOI] [PubMed] [Google Scholar]
- Jones WG, Fossa SD, Mead GM, Roberts JW, Sokal M, Horwich A, Stenning SP. Randomized trial of 30 versus 20 Gy in the adjuvent treatment of stage I testicular seminoma. A report on medical research conical trial TE18, European Organization for the research and treatment of cancer trial 30942 (ISRCTN18525328) J Clin Oncol. 2005;23:1200–1208. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kayl AE, Meyers CA. Side effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol. 2006;18:24–28. doi: 10.1097/01.gco.0000192996.20040.24. [DOI] [PubMed] [Google Scholar]
- King GL. Characterization of radiation-induced emesis in the ferret. Radiat Res. 1988;114:599–612. [PubMed] [Google Scholar]
- King GL. Animal models in the study of vomiting. Can J Physiol Pharmacol. 1990;68:260–268. doi: 10.1139/y90-040. [DOI] [PubMed] [Google Scholar]
- King GL, Landauer MR. Effects of zacopride and BMY25801 (Batanopride) on radiation-induced emesis and locomotor behavior in the ferret. J Pharmacol Exp Therap. 1990;235:1026–1033. [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (House musk shrew) Psychopharmacology. 2004;147:254–259. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- London SW, McCarthy LE, Borrison HL. Suppression of cancer chemotherapy-induced vomiting in the cat by nabilone, a synthetic cannabinoid. Proc Soc Exp Biol Med. 1979;160:437–440. doi: 10.3181/00379727-160-40465. [DOI] [PubMed] [Google Scholar]
- Lucraft HH, Palmer MK. Randomized clinical trials of levonantradol and chlorpromazine in the prevention of radiotherapy-induced vomiting. Clin Radiol. 1982;33:621–622. doi: 10.1016/s0009-9260(82)80383-8. [DOI] [PubMed] [Google Scholar]
- Maranzano E. Radiation-induced emesis: A problem with many open questions. Tumori. 2001;87:213–218. doi: 10.1177/030089160108700401. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Feyer PC, Molassiotis A, Rossi R, Clark-Snow RA, Olver I, Warr D, Schiavone C, Roila R. Evidence based recommendations for the use of antiemetics in radiotherapy. Radiotherapy and Oncology. 2005;76:227–233. doi: 10.1016/j.radonc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, Leonard CM, Chirwa SS, Gong J, Uh GR. Endocannabinoid receptor genetics and marijuana use . In: Onaivi ES, Sugiura T, Di Marzo V, editors. Endocannabinoids. The brain and body’s marijuana and beyond. Vol. 118 CRC/Taylor and Francis; Boca Raton: 2006. p. 57. [Google Scholar]
- Osoba D, Zee B, Warr D, Kaizer L, Latreille J, Pater J. Quality of Life Studies in Chemotherapy-induced emesis. Oncology. 1996;5:92–95. doi: 10.1159/000227647. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabis and cannabinoids: Pharmacology and rational clinical use. Forsch Komplement. 1999;3:12–15. doi: 10.1159/000057150. [DOI] [PubMed] [Google Scholar]
- Preziosi PD’, Amato M, Carmine RD, Martire M, Pozzoli G, Navara P. The effects of 5-HT3 receptor antagonists on cisplatin-induced emesis in the pigeon. Eur J Pharmacol. 1992;221:343–350. doi: 10.1016/0014-2999(92)90721-f. [DOI] [PubMed] [Google Scholar]
- Priestman SG, Priestman TG, Canny PA. A double-blind randomized cross-over comparison of nabilone and metoclopramide in the control of radiation. Clin Radiol. 1987;38:543–544. doi: 10.1016/s0009-9260(87)80151-4. [DOI] [PubMed] [Google Scholar]
- Razdan R. Structure-activity relationships in cannabinoids. Pharmacol Revs. 1986;38:75–149. [PubMed] [Google Scholar]
- Sam TSW, Cheng JTY, Johnston KD, Kan KKW, Ngan NP, Rudd JA, Wai NK, Yeung JHK. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus marinus (house musk shrew) Eur J Pharmacol. 2003;472:135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- Scarantino CW, Ornitz RD, Hoffman LG, Anderson RF., Jr On the mechanism of radiation-induced emesis: the role of serotonin. Int J Radiat Oncol Biol Phys. 1994;30:825–830. doi: 10.1016/0360-3016(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy. A comparison of oral delta-9-THC and prochlorperazine. Cancer. 1982;50:636–645. doi: 10.1002/1097-0142(19820815)50:4<636::aid-cncr2820500404>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Van der Meeren A, Lebaron-Jacobs L. Behavioral consequences of an 8 Gy total body radiation in mice: regulation by interleukin 4. Can J Physiol. 2001;79:140–143. [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey K. Identification and functional characterization of brainstem cannabinoid CB2receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA. Cannabinoids inhibit emesis through CB1receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta-9- tetrahydrocannabinol selectively acts on CB1receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285 :G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy. Drugs. 1997;53:206–234. doi: 10.2165/00003495-199753020-00003. [DOI] [PubMed] [Google Scholar]


