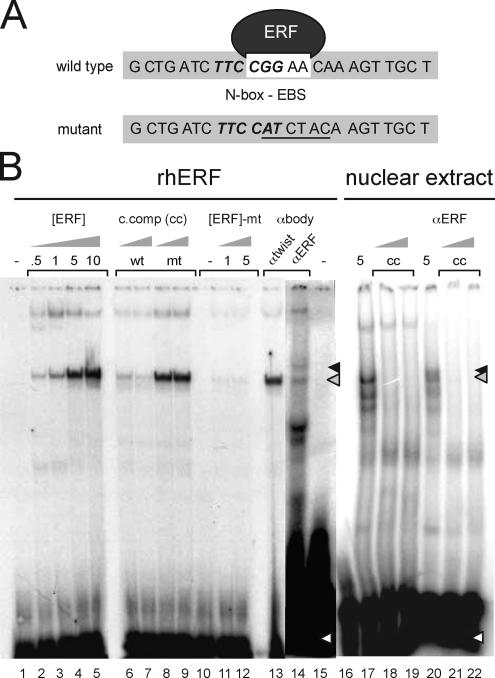
Figure 1.
ERF binds to the utrophin-A promoter N-box/EBS element. (A) Sequence of the human utrophin-A promoter probes containing the N-box/EBS motif used in gel shifts. The N-box is in bold italics; the EBS is in a white box. Mutant refers to the gel shift probe used that contains a mutant N-box/EBS site. (B) The N-box/EBS site in the human utrophin A promoter binds ERF. rhERF was in vitro synthesized and 0.5–10 μl (0.5–10; lanes 2–5) was incubated with the labeled oligonucleotide probe. Five microliters each of C2C12 extract was used for analysis of endogenous ERF in lanes 17–22. Formation of a single specific DNA–protein complex (gray arrow) was observed with rhERF (lanes 2–5), which could be specifically competed away with 10- and 100-fold excess of wild-type cold competitor oligonucleotide (lanes 6 and 7) but not the mutant probe (lanes 8 and 9). rhERF did not bind a double-stranded probe of the same region with a mutated N-box/EBS site (lanes 10–12). The black arrow indicates ERF–DNA complex supershifted in the presence of 5 μl of ERF-specific antibodies (lane 14) but not in the presence of a nonspecific antibody against the transcription factor Twist (lane 13). White arrows represent free radiolabeled probe. Wt, wild-type probe; mt, N-box/EBS mutant probe; [ERF], amount of rhERF protein in microliters; c, comp, unlabeled N-box/EBS oligonucleotide; αERF, ERF S17C rabbit polyclonal antibody.